Short Answer
Classify the compound below as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network. 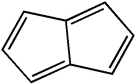
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q6: Is the all-cis form of [10]annulene aromatic?
Q11: Which of the following structures, if flat,
Q13: Provide an acceptable name for the compound
Q16: Classify the compound below as aromatic, antiaromatic,
Q19: Classify the compound below as aromatic, antiaromatic,
Q58: Name two of the three common allotropes
Q99: Which of the following is not a
Q105: When cycloheptatriene is deprotonated, an anion with
Q108: Provide the structure of 2,4,6-trichlorophenol.
Q119: Provide the structure of m-xylene.