Short Answer
Classify the compound below as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network. 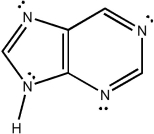
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q11: Provide the structure of sodium cyclopentadienide.
Q34: Provide the structure of 2,5-dichlorophenol.
Q35: Which of the following is an incorrect
Q45: How many pairs of degenerate π molecular
Q69: Provide the structure of 2-bromo-4-chlorobenzoic acid.
Q70: Provide the structure of m-nitrophenol.
Q83: Nitrogen's lone pair electrons occupy what type
Q91: Which of the following is also an
Q92: What is suggested by the fact that
Q114: Classify the compound below as aromatic, antiaromatic,