Multiple Choice
When 1 mole of anhydrous HCl is reacted with excess 1,3-pentadiene, both the 1,2 and the 1,4-addition products are formed. Which of the following structures shown below is the least likely to be one of these products? (Note: When a chiral carbon is formed in this reaction a racemic mixture results, only one of the two possible enantiomers is shown.)
A) 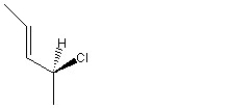
B) 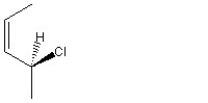
C) 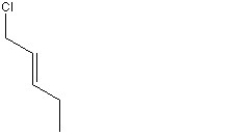
D) 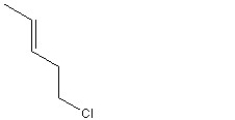
E) 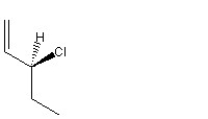
Correct Answer:

Verified
Correct Answer:
Verified
Q8: Which diene reacts more rapidly in Diels-Alder
Q37: Draw (Z)-1,3-hexadiene in its s-trans conformation.
Q58: What name is given to the type
Q64: Draw the LUMO of pentadienyl cation.
Q71: When (S)-3-bromopent-1-ene is heated in water, which
Q85: What characterizes a pericyclic reaction?
Q109: In the planar conformation of 1,3-butadiene, the
Q111: Indicate whether the following cyclization, conducted under
Q112: Provide the structure of the major product
Q118: Provide the structure of the major organic