Multiple Choice
According to the 1,3-butadiene structure below, which positions would be best to place methoxy groups to yield the most reactive dimethoxy-1,3-butadiene isomer in the Diels-Alder reaction? 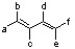
A) c and d
B) a and c
C) b and c
D) a and d
E) a and f
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q24: Which sequence correctly ranks the following dienes
Q28: What is the resonance energy of a
Q29: Provide the major organic product of the
Q30: Provide the structure of the major organic
Q31: Provide the major organic product of the
Q32: Provide the structure of the major organic
Q33: Draw the s-cis conformation of (E)-1,3-hexadiene.
Q65: Draw the HOMO of pentadienyl anion.
Q72: Is the thermal [4+2] cycloaddition between allyl
Q109: What is the hybridization of the central