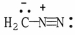Related Questions
Q47: Strong bases usually contain positively charged atoms
Q48: Within a given row of the periodic
Q54: Which of the following condensed formulas represents
Q57: Draw a correct Lewis structure for tert-butyl
Q60: The compound methylamine, CH<sub>3</sub>NH<sub>2</sub>, contains a C-N
Q62: Use the curved arrow formalism to indicate
Q63: Add the appropriate formal charge to each
Q94: Consider the set of compounds, NH<sub>3</sub>, HF,
Q113: The electronegativity of elements on the periodic
Q121: What is the pK<sub>a</sub> and general acid