Essay
Draw 3 significant resonance structures for the compound shown below. Place a box around the major contributor. Fill in any missing formal charges. 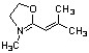
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q13: When a negatively charged species is most
Q17: Provide the line-angle formula for the alcohol
Q19: According to the Lewis definition, which of
Q35: Calculate the pH of a 100 mL
Q39: The Lewis structure of trimethylamine is shown
Q45: Indicate the line-angle structure that corresponds to
Q58: The electron density of _ orbitals has
Q75: Which of the following acids has the
Q104: The _ tells us that each orbital
Q119: Which of the following compounds are covalent