Multiple Choice
Below is a representation of pure liquid HX,an amphoteric substance,at equilibrium.(Each circle represents 1.0 ×10-3 mol of atoms,and the volume of the box is 1.0 L.) 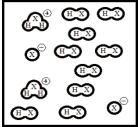 What is the autoionization constant for HX?
What is the autoionization constant for HX?
A) 4.9 ×10-2
B) 4.0 ×10-3
C) 4.4 ×10-4
D) 4.0 ×10-6
E) 2.5 ×105
Correct Answer:

Verified
Correct Answer:
Verified
Q21: The most basic oxides are formed from
Q43: Acid strength decreases in the series HI
Q44: Which of the following is the correct
Q45: Calculate the concentration of malonate ion (C<sub>3</sub>H<sub>2</sub>O<sub>4</sub><sup>2</sup><sup>-</sup>)in
Q49: What is [OH<sup>-</sup>] for a solution at
Q52: Which is a Lewis acid but not
Q75: Amphoteric oxides exhibit both acidic and basic
Q75: Equal volumes of two solutions, one containing
Q109: Picric acid has been used in the
Q142: Since zirconium is a metal, ZrO<sub>2</sub> is