Multiple Choice
Which represents a weak base B with Kb= 2 ×10-3? (Each circle represents 1.0 ×10-3 mol of atoms,and the volume of each box is 1.0 L.Solvent water molecules are not shown for clarity.)
A) 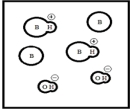
B) 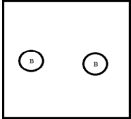
C) 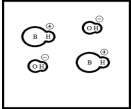
D) 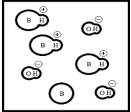
E) 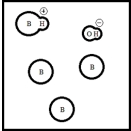
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Amphoteric oxides are compounds that exhibit both
Q13: What is the conjugate base of water?<br>A)
Q20: What is the conjugate base of HSO<sub>4</sub><sup>-</sup>
Q21: Consider the weak bases below and their
Q25: What is [OH<sup>-</sup>] for a solution at
Q26: K<sub>w</sub> = 1.0 × 10<sup>-</sup><sup>14</sup> under all
Q28: Below is a representation of an aqueous
Q29: In the reaction CaO(s)+ SO<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6485/.jpg"
Q39: Which one of the following is a
Q81: Which aqueous solution has the highest pH?<br>A)