Multiple Choice
Below is a diagram representing a solvent A(l) in a 1-L beaker,and a solute X dissolved in the solvent.Solvent A has a density of 0.8 g/mL,and a molar mass of 40 g/mol. Solute X has a molar mass of 30 g/mol.Each circle of X represents 1 mol of X.Assume that the solute addition does not significantly change the volume of liquid in the beaker. 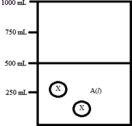 What is the molality of the solute X in this solution?
What is the molality of the solute X in this solution?
A) 2 m
B) 4 m
C) 5 m
D) 0.004 m
E) 0.005 m
Correct Answer:

Verified
Correct Answer:
Verified
Q1: What is the name of the process
Q29: _ is the process used to stabilize
Q37: Potassium fluoride is used for frosting glass.
Q70: Which statement is false?<br>A) The vapor pressure
Q84: The _ _ is the scattered light
Q95: Select the strongest electrolyte from the following
Q116: What is the percent CdSO<sub>4</sub> by mass
Q118: Benzaldehyde (106.12 g/mol), also known as oil
Q130: What mass of water is required to
Q132: Which solution has the highest vapor pressure?<br>A)0.75