Multiple Choice
Below is a diagram representing a solvent A(l) in a 1-L beaker,and a solute X dissolved in the solvent.Solvent A has a density of 0.8 g/mL,and a molar mass of 40 g/mol. Solute X has a molar mass of 30 g/mol.Each circle of X represents 1 mol of X.Assume that the solute addition does not significantly change the volume of liquid in the beaker. 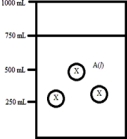 What is the mass percent of solute X in this solution?
What is the mass percent of solute X in this solution?
A) 0.4%
B) 3%
C) 12%
D) 13%
E) 20%
Correct Answer:

Verified
Correct Answer:
Verified
Q12: A hydrophilic colloid must generally be stabilized.
Q25: What is the name given to the
Q26: Explain the following, on the basis of
Q30: Which process defines how molecular compounds form
Q32: A 0.100 m MgSO<sub>4</sub> solution has a
Q57: What is the vapor pressure above a
Q59: Determine the freezing point of a solution
Q62: Carbon tetrachloride,once widely used in fire extinguishers
Q90: Which of the following aqueous solutions should
Q128: Hydration is the process in which organic