Multiple Choice
Below is a diagram representing two chambers containing solvent A,separated by a semipermeable membrane (a dashed line) which allows only solvent A to pass through. 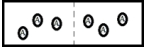 If a solute X is added to the chamber on the left,which diagram below best represents how the system will respond in order to reach a new equilibrium?
If a solute X is added to the chamber on the left,which diagram below best represents how the system will respond in order to reach a new equilibrium?
A) 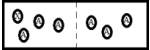
B) 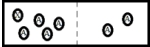
C)  C
C
D) 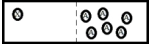
E) 
Correct Answer:

Verified
Correct Answer:
Verified
Q6: If a solute dissolves in an endothermic
Q21: From the following list of aqueous solutions
Q24: If a 100-mL sample of a volatile
Q39: The density of a 20.3 M CH<sub>3</sub>OH
Q42: Osmosis is the selective passage of solvent
Q57: The solubility of the oxidizing agent potassium
Q91: What mass of ethanol, C<sub>2</sub>H<sub>5</sub>OH a nonelectrolyte,
Q121: What is the freezing point of a
Q129: When a nonvolatile solute is dissolved in
Q151: What term describes the process when two