Multiple Choice
Below is the phase diagram for sulfur.Which phase has the highest density at 119°C? 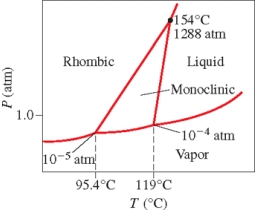
A) liquid
B) vapor
C) rhombic solid
D) monoclinic solid
E) liquid-vapor boundary line
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q20: In the packing of identical atoms with
Q23: Solids are generally most stable in crystalline
Q30: Only molecules which do not have dipole
Q34: What types of intermolecular forces exist between
Q36: Which kinds of intermolecular forces exist between
Q40: Which of the following pure substances has
Q41: Which of the following lacks a regular
Q58: For which of the following pure substances
Q85: Ethanol (C<sub>2</sub>H<sub>5</sub> - OH) will have a
Q130: _ _ are solids that lack a