Multiple Choice
Based on the phase diagram of a pure substance given below,what is the significance of the point labeled B? 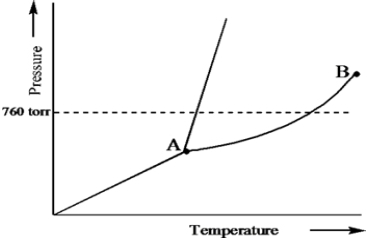
A) It is the normal melting point.
B) It is the triple point.
C) It is the critical point.
D) It is the normal sublimation point.
E) It is the normal boiling point.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Liquid sodium can be used as a
Q4: Molecular crystals are held together by the
Q21: Helium atoms do not combine to form
Q42: Identify the dominant (strongest)type of intermolecular force
Q65: The atomic planes in a graphite crystal
Q79: Which one of the following substances crystallizes
Q95: If a molecule at the surface of
Q105: Krypton has a higher melting point than
Q118: a.State the essential requirements for hydrogen bonding
Q123: For the solid forms of the following