Multiple Choice
Based on the phase diagram of a pure substance given below,what change of state occurs as the substance changes from point C to point D? 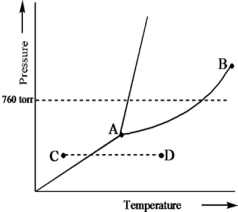
A) The substance increases in temperature with a phase change from solid to liquid.
B) The substance increases in temperature with a phase change from solid to vapor.
C) The substance increases in temperature with a phase change from liquid to vapor.
D) The substance increases in temperature but does not undergo a phase change.
E) The substance increases in temperature and becomes a supercritical fluid.
Correct Answer:

Verified
Correct Answer:
Verified
Q7: What is another name given to dispersion
Q27: Which one of the following crystallizes in
Q41: Which one of the following pure substance
Q42: Identify the dominant (strongest)type of intermolecular force
Q81: One of the crystalline forms of zinc
Q93: Octane, C<sub>8</sub>H<sub>18</sub>, boils at 125°C, whereas water
Q109: Which one of the following involves ion-dipole
Q118: a.State the essential requirements for hydrogen bonding
Q123: For the solid forms of the following
Q125: What is defined as the number of