Multiple Choice
Below is a representation of liquid water in equilibrium with its water vapor in a rigid container at 35ºC.The circles represent water vapor. 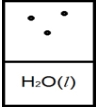 Which diagram below best represents liquid water in equilibrium with its water vapor at 70ºC? The heat of vaporization of water is 40.7 kJ/mol.(R = 8.314 J/K • mol)
Which diagram below best represents liquid water in equilibrium with its water vapor at 70ºC? The heat of vaporization of water is 40.7 kJ/mol.(R = 8.314 J/K • mol)
A) 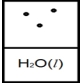
B) 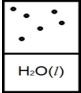
C) 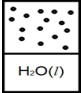
D) 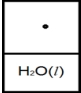
E) 
Correct Answer:

Verified
Correct Answer:
Verified
Q49: Diethyl ether,(CH<sub>3</sub>CH<sub>2</sub>)<sub>2</sub>O,used as a solvent for extraction
Q49: Naphthalene sublimes at room temperature and 1
Q52: A metal such as chromium in the
Q55: The arrow in this phase diagram represents
Q56: In which of the following compounds will
Q68: _ _ is a special type of
Q93: The maximum number of phases of a
Q125: Which of the following statements is true?<br>A)
Q127: What quantity of heat is required to
Q146: Identify the dominant (strongest)type of intermolecular force