Multiple Choice
Based on the phase diagram of a pure substance given below,which statement is true? 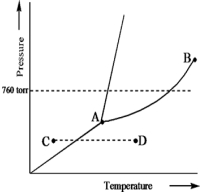
A) The solid phase has a lower density than the liquid phase.
B) The triple point is at a higher temperature than the normal melting point.
C) The substance changes from a solid to a liquid as one follows the line from C to D.
D) The substance changes from a liquid to a gas as one follows the line from C to D.
E) Point B represents the critical temperature and pressure for the substance.
Correct Answer:

Verified
Correct Answer:
Verified
Q9: What name is given to the curved
Q27: The strongest intermolecular interactions between pentane (C<sub>5</sub>H<sub>12</sub>)
Q44: Which substance should exhibit hydrogen bonding in
Q50: The strongest intermolecular interactions between hydrogen sulfide
Q82: The surface tension of water is lowered
Q92: Select the pair of substances in which
Q97: Based on the phase diagram of a
Q99: What is the attractive force between like
Q100: Which is not a valid phase diagram
Q108: The number of atoms in a face-centered