Essay
Consider the phase diagram shown below. 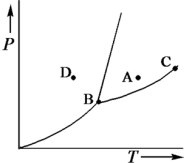 a.What phase(s)is/are present at point A?
a.What phase(s)is/are present at point A?
b.What phase(s)is/are present at point B?
c.Name point C and explain its significance.
d.Starting at D,if the pressure is lowered while the temperature remains constant,describe what will happen.
Correct Answer:

Verified
a.liquid b.solid,liquid,and gas c.C is t...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q3: _ is the name given to the
Q7: What is another name given to dispersion
Q26: The strongest intermolecular interactions between hydrogen fluoride
Q27: Which one of the following crystallizes in
Q80: Which substance has the highest vapor pressure
Q89: Vanadium crystallizes in a body-centered cubic lattice,
Q109: Which one of the following involves ion-dipole
Q115: The vapor pressure of ethanol is 400.
Q125: What is defined as the number of
Q134: Which has the highest surface tension at