Multiple Choice
Which of these Lewis structures is incorrect?
A) 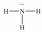
B) 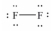
C) 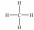
D) 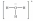
E) 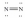
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: Which of these substances will display an
Q28: Ignoring resonance, the formal charge on the
Q52: Which of the following contains covalent bonds?<br>A)BaO<br>B)IBr<br>C)Mg<br>D)LiBr<br>E)Cu
Q52: In the best Lewis structure for the
Q56: How many lone pairs of electrons need
Q61: How many dots does the Lewis dot
Q66: In Ba(CN)<sub>2</sub>, the bonding is<br>A) essentially ionic.<br>B)
Q72: Which molecule has a Lewis structure that
Q75: Ionic compounds tend to form between metals
Q100: A(n) _ is a representation of covalent