Multiple Choice
The formal charge on Cl in the structure shown for the perchlorate ion is _____. 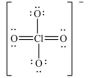
A) -2
B) -1
C) 0
D) +1
E) +2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: Which of the following contains ionic bonding?<br>A)CO<br>B)SrF
Q8: What formal charge resides on the carbon
Q14: The Lewis dot symbol consists of the
Q43: The number of resonance structures for the
Q52: In the best Lewis structure for the
Q61: How many dots does the Lewis dot
Q63: Select the Lewis structure for XeO<sub>2</sub>F<sub>2</sub> which
Q71: Which molecule has the largest dipole moment?<br>A)
Q75: Ionic compounds tend to form between metals
Q124: The total number of bonding electrons in