Multiple Choice
Suppose aqueous solutions of silver(I) nitrate and potassium chloride are mixed.Which represents the likely result?
A) 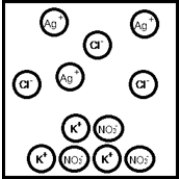
B) 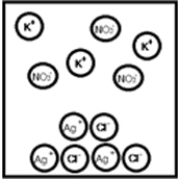
C) 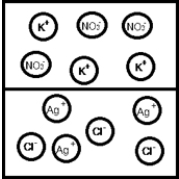
D) 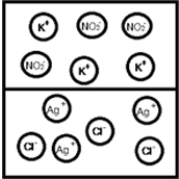
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q10: The distinguishing characteristic of all electrolyte solutions
Q13: How many moles of H<sup>+</sup>(aq) ions are
Q16: Complete the following reaction and identify the
Q29: Which of the following is soluble in
Q33: Which of these compounds is a strong
Q56: For the reaction depicted below,if Z represents
Q76: Which of these equations does not represent
Q107: Which process defines how an ionic compounds
Q119: What is oxidized in the following reaction?
Q144: The following reaction will occur Na(s) +