Multiple Choice
Which is a representation of a balanced chemical equation for the reaction of nitrogen gas and chlorine gas to form nitrogen trichloride?
A) 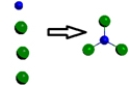
B) 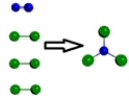
C) 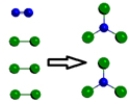
D) 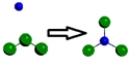
E) 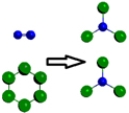
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q4: Once the following equation is balanced with
Q22: What is the average mass, in grams,
Q40: Lead(II) sulfide was once used in glazing
Q43: Calculate the molar mass of sulfuric acid.<br>A)
Q44: The _ is the amount of product
Q67: Aluminum metal reacts with chlorine gas to
Q106: What is the percent carbon in CH<sub>3</sub>CH<sub>2</sub>OH?<br>A)
Q116: What is the mass of 1.21 ×
Q132: Potassium chloride is used as a substitute
Q141: How many grams of water could be