Multiple Choice
Suppose white atoms (W) and black atoms (B) react according to the following balanced chemical equation. 2W + B → BW2
If the initial reaction mixture is prepared as follows,what will the final reaction mixture be once the reaction is complete? 
A) 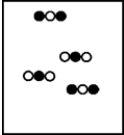
B) 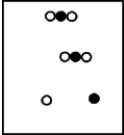
C) 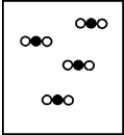
D) 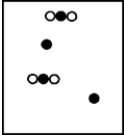
E) 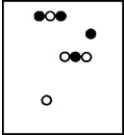
Correct Answer:

Verified
Correct Answer:
Verified
Q15: The empirical formula of C<sub>6</sub>H<sub>6</sub> is CH.
Q51: Once the following equation is balanced with
Q59: Phosphorus pentachloride, a white solid that has
Q85: What is the name given to the
Q95: Calculate the formula mass of rubidium carbonate,
Q103: Calculate the formula mass of (NH<sub>4</sub>)<sub>3</sub>AsO<sub>4</sub>.<br>A)417.80 amu<br>B)193.05
Q104: Once the following equation is balanced with
Q107: What mass of excess reactant remains at
Q110: What is the mass,in grams,of one arsenic
Q122: Ammonia reacts with diatomic oxygen to form