Deck 39: Wave Functions and Uncertainty
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/18
Play
Full screen (f)
Deck 39: Wave Functions and Uncertainty
1
Find the value of A to normalize the wave function ψ(x)= 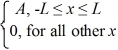 .
.
A)1/L
B)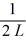
C)1/L2
D)1.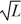
E)1/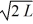
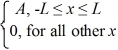 .
.A)1/L
B)
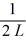
C)1/L2
D)1.
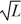
E)1/
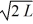
1/ 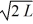
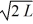
2
A particle is confined to a one-dimensional box (an infinite well)on the x-axis between x = 0 and x = L.The potential height of the walls of the box is infinite.The normalized wave function of the particle,which is in the ground state,is given by ψ(x)= 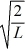 sin
sin 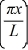 ,with 0 ≤ x ≤ L.What is the probability of finding the particle between x = 0 and x = L/3?
,with 0 ≤ x ≤ L.What is the probability of finding the particle between x = 0 and x = L/3?
A)0.20
B)0.22
C)0.24
D)0.26
E)0.28
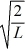 sin
sin 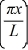 ,with 0 ≤ x ≤ L.What is the probability of finding the particle between x = 0 and x = L/3?
,with 0 ≤ x ≤ L.What is the probability of finding the particle between x = 0 and x = L/3?A)0.20
B)0.22
C)0.24
D)0.26
E)0.28
0.20
3
A measurement of an electron's speed is 2.0 × 106 m/s and has an uncertainty of 10%.What is the minimum uncertainty in its position? (h = 6.626 × 10-34 J ∙ s,mel = 9.11 × 10-31 kg)
A)0.91 nm
B)1.8 nm
C)2.7 nm
D)2.8 nm
E)5.0 nm
A)0.91 nm
B)1.8 nm
C)2.7 nm
D)2.8 nm
E)5.0 nm
1.8 nm
4
The probability density for an electron that has passed through an experimental apparatus is shown in the figure.If 4100 electrons pass through the apparatus,what is the expected number that will land in a 0.10 mm-wide strip centered at x = 0.00 mm? 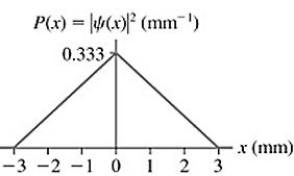
A)140
B)1400
C)450
D)45

A)140
B)1400
C)450
D)45

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
5
An electron inside a hydrogen atom is confined to within a space of 0.110 nm.What is the minimum uncertainty in the electron's velocity? (h = 6.626 × 10-34 J ∙ s,mel = 9.11 × 10-31 kg)
A)3.31 × 106 m/s
B)4.71 × 106 m/s
C)3.30 × 108 m/s
D)4.71 × 108 m/s
E)3.30 × 1010 m/s
A)3.31 × 106 m/s
B)4.71 × 106 m/s
C)3.30 × 108 m/s
D)4.71 × 108 m/s
E)3.30 × 1010 m/s

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
6
A nonrelativistic proton is confined to a length of 2.0 pm on the x-axis.What is the kinetic energy of the proton if its speed is equal to the minimum uncertainty possible in its speed? (1 eV = 1.60 × 10-19 J,h = 6.626 × 10-34 J ∙ s,mproton = 1.67 × 10-27 kg)
A)5.1 eV
B)51 eV
C)510 eV
D)5100 eV
E)51,000 eV
A)5.1 eV
B)51 eV
C)510 eV
D)5100 eV
E)51,000 eV

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
7
A particle is confined to a one-dimensional box (an infinite well)on the x-axis between x = 0 and x = L.The potential height of the walls of the box is infinite.The normalized wave function of the particle,which is in the ground state,is given by ψ(x)= 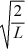 sin
sin 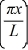 ,with 0 ≤ x ≤ L.What is the maximum probability per unit length of finding the particle?
,with 0 ≤ x ≤ L.What is the maximum probability per unit length of finding the particle?
A)1/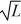
B)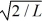
C)2/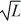
D)1/L
E)2/L
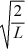 sin
sin 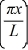 ,with 0 ≤ x ≤ L.What is the maximum probability per unit length of finding the particle?
,with 0 ≤ x ≤ L.What is the maximum probability per unit length of finding the particle?A)1/
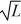
B)
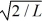
C)2/
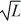
D)1/L
E)2/L

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
8
The wave function for an electron that is confined to x ≥ 0 nm is
ψ(x)=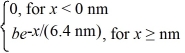 a)What must be the value of b?
a)What must be the value of b?
(b)What is the probability of finding the electron in a 0.010 nm-wide region centered at x = 1.0 nm?
ψ(x)=
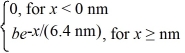 a)What must be the value of b?
a)What must be the value of b?(b)What is the probability of finding the electron in a 0.010 nm-wide region centered at x = 1.0 nm?

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
9
If the accuracy in measuring the velocity of a particle increases,the accuracy in measuring its position will
A)increase.
B)decrease.
C)remain the same.
D)It is impossible to say since the two measurements are independent and do not affect each other.
A)increase.
B)decrease.
C)remain the same.
D)It is impossible to say since the two measurements are independent and do not affect each other.

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
10
A molecule of roughly spherical shape has a mass of 6.10 × 10-25 kg and a diameter of 0.70 nm.The uncertainty in the measured position of the molecule is equal to the molecular diameter.What is the minimum uncertainty in the speed of this molecule? (h = 6.626 × 10-34 J ∙ s)
A)0.78 m/s
B)7.8 m/s
C)78 m/s
D)0.078 m/s
E)0.0078 m/s
A)0.78 m/s
B)7.8 m/s
C)78 m/s
D)0.078 m/s
E)0.0078 m/s

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
11
If the accuracy in measuring the position of a particle increases,the accuracy in measuring its velocity will
A)increase.
B)decrease.
C)remain the same.
D)It is impossible to say since the two measurements are independent and do not affect each other.
A)increase.
B)decrease.
C)remain the same.
D)It is impossible to say since the two measurements are independent and do not affect each other.

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
12
The wave function for an electron that is confined to x ≥ 0 nm is
ψ(x)=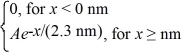 (a)What must be the value of A?
(a)What must be the value of A?
(b)What is the probability of finding the electron in the interval 1.15 nm ≤ x ≤ 1.84 nm?
ψ(x)=
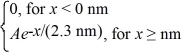 (a)What must be the value of A?
(a)What must be the value of A?(b)What is the probability of finding the electron in the interval 1.15 nm ≤ x ≤ 1.84 nm?

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
13
A set of five possible wave functions is given below,where L is a positive real number. ψ1(x)= Ae-x,for all x ψ2(x)= A cos x,for all x
Ψ3(x)=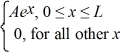 ψ4(x)=
ψ4(x)= 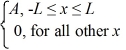 ψ5(x)=
ψ5(x)= 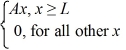 Which of the five possible wave functions are normalizable? (There may be more than one correct choice.)
Which of the five possible wave functions are normalizable? (There may be more than one correct choice.)
A)ψ1(x)
B)ψ2(x)
C)ψ3(x)
D)ψ4(x)
E)ψ5(x)
Ψ3(x)=
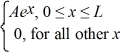 ψ4(x)=
ψ4(x)= 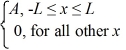 ψ5(x)=
ψ5(x)= 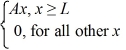 Which of the five possible wave functions are normalizable? (There may be more than one correct choice.)
Which of the five possible wave functions are normalizable? (There may be more than one correct choice.)A)ψ1(x)
B)ψ2(x)
C)ψ3(x)
D)ψ4(x)
E)ψ5(x)

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
14
A nonrelativistic electron is confined to a length of 500 pm on the x-axis.What is the kinetic energy of the electron if its speed is equal to the minimum uncertainty possible in its speed? (h = 6.626 × 10-34 J ∙ s,mel = 9.11 × 10-31 kg,1 eV = 1.60 × 10-19 J)
A)0.015 eV
B)0.15 eV
C)1.5 eV
D)15 eV
E)150 eV
A)0.015 eV
B)0.15 eV
C)1.5 eV
D)15 eV
E)150 eV

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
15
The wave function for a particle must be normalizable because
A)the particle's momentum must be conserved.
B)the particle's angular momentum must be conserved.
C)the particle's charge must be conserved.
D)the particle must be somewhere.
E)the particle cannot be in two places at the same time.
A)the particle's momentum must be conserved.
B)the particle's angular momentum must be conserved.
C)the particle's charge must be conserved.
D)the particle must be somewhere.
E)the particle cannot be in two places at the same time.

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
16
A small dust particle of mass 7.90 × 10-6 g is being observed under a magnifying lens.Its position is determined to within 0.0050 mm.(1 y = 3.156 × 107 s,h = 6.626 × 10-34 J ∙ s)
(a)Find the minimum uncertainty in its velocity implied by the uncertainty in its position.
(b)Assuming the dust particle is moving at the speed you just found,how many years would it take for the particle to move 1.0 mm?
(a)Find the minimum uncertainty in its velocity implied by the uncertainty in its position.
(b)Assuming the dust particle is moving at the speed you just found,how many years would it take for the particle to move 1.0 mm?

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
17
The square of the wave function of a particle,|ψ(x)|2,gives the probability of finding the particle at the point x.

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
18
Find the value of A to normalize the wave function ψ(x)= 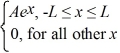 .
.
A)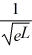
B)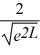
C)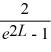
D)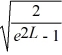
E)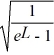
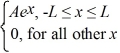 .
.A)
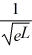
B)
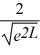
C)
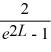
D)
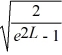
E)
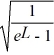

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck


