Exam 39: Wave Functions and Uncertainty
Exam 1: Concepts of Motion52 Questions
Exam 2: Kinematics in One Dimension59 Questions
Exam 3: Vectors and Coordinate Systems33 Questions
Exam 4: Kinematics in Two Dimensions49 Questions
Exam 5: Force and Motion30 Questions
Exam 6: Dynamics I: Motion Along a Line46 Questions
Exam 7: Newtons Third Law43 Questions
Exam 8: Dynamics II: Motion in a Plane20 Questions
Exam 9: Work and Kinetic Energy66 Questions
Exam 10: Interactions and Potential Energy55 Questions
Exam 11: Impulse and Momentum43 Questions
Exam 12: Rotation of a Rigid Body116 Questions
Exam 13: Newtons Theory of Gravity50 Questions
Exam 14: Fluids and Elasticity72 Questions
Exam 15: Oscillations49 Questions
Exam 16: Traveling Waves51 Questions
Exam 17: Superposition51 Questions
Exam 18: A Macroscopic Description of Matter46 Questions
Exam 19: Work, heat, and the First Law of Thermodynamics96 Questions
Exam 20: The Micromacro Connection41 Questions
Exam 21: Heat Engines and Refrigerators44 Questions
Exam 22: Electric Charges and Forces26 Questions
Exam 23: The Electric Field32 Questions
Exam 24: Gausss Law41 Questions
Exam 25: The Electric Potential40 Questions
Exam 26: Potential and Field57 Questions
Exam 27: Current and Resistance32 Questions
Exam 28: Fundamentals of Circuits68 Questions
Exam 29: The Magnetic Field83 Questions
Exam 30: Electromagnetic Induction66 Questions
Exam 31: Electromagnetic Fields and Waves52 Questions
Exam 32: Ac Circuits44 Questions
Exam 33: Wave Optics51 Questions
Exam 34: Ray Optics60 Questions
Exam 35: Optical Instruments52 Questions
Exam 36: Relativity49 Questions
Exam 37: The Foundations of Modern Physics7 Questions
Exam 38: Quantization45 Questions
Exam 39: Wave Functions and Uncertainty18 Questions
Exam 40: One-Dimensional Quantum Mechanics32 Questions
Exam 41: Atomic Physics38 Questions
Exam 42: Nuclear Physics64 Questions
Select questions type
Find the value of A to normalize the wave function ψ(x)= 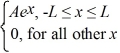 .
.
Free
(Multiple Choice)
4.7/5  (30)
(30)
Correct Answer:
D
A nonrelativistic electron is confined to a length of 500 pm on the x-axis.What is the kinetic energy of the electron if its speed is equal to the minimum uncertainty possible in its speed? (h = 6.626 × 10-34 J ∙ s,mel = 9.11 × 10-31 kg,1 eV = 1.60 × 10-19 J)
Free
(Multiple Choice)
4.9/5  (34)
(34)
Correct Answer:
C
A measurement of an electron's speed is 2.0 × 106 m/s and has an uncertainty of 10%.What is the minimum uncertainty in its position? (h = 6.626 × 10-34 J ∙ s,mel = 9.11 × 10-31 kg)
Free
(Multiple Choice)
4.9/5  (40)
(40)
Correct Answer:
B
The wave function for an electron that is confined to x ≥ 0 nm is
ψ(x)= 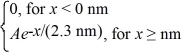 (a)What must be the value of A?
(b)What is the probability of finding the electron in the interval 1.15 nm ≤ x ≤ 1.84 nm?
(a)What must be the value of A?
(b)What is the probability of finding the electron in the interval 1.15 nm ≤ x ≤ 1.84 nm?
(Short Answer)
4.9/5  (40)
(40)
A small dust particle of mass 7.90 × 10-6 g is being observed under a magnifying lens.Its position is determined to within 0.0050 mm.(1 y = 3.156 × 107 s,h = 6.626 × 10-34 J ∙ s)
(a)Find the minimum uncertainty in its velocity implied by the uncertainty in its position.
(b)Assuming the dust particle is moving at the speed you just found,how many years would it take for the particle to move 1.0 mm?
(Essay)
4.8/5  (30)
(30)
A set of five possible wave functions is given below,where L is a positive real number. ψ1(x)= Ae-x,for all x ψ2(x)= A cos x,for all x
Ψ3(x)= 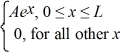 ψ4(x)=
ψ4(x)= 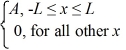 ψ5(x)=
ψ5(x)= 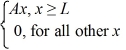 Which of the five possible wave functions are normalizable? (There may be more than one correct choice.)
Which of the five possible wave functions are normalizable? (There may be more than one correct choice.)
(Multiple Choice)
4.8/5  (30)
(30)
A particle is confined to a one-dimensional box (an infinite well)on the x-axis between x = 0 and x = L.The potential height of the walls of the box is infinite.The normalized wave function of the particle,which is in the ground state,is given by ψ(x)= 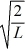 sin
sin 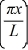 ,with 0 ≤ x ≤ L.What is the probability of finding the particle between x = 0 and x = L/3?
,with 0 ≤ x ≤ L.What is the probability of finding the particle between x = 0 and x = L/3?
(Multiple Choice)
4.9/5  (34)
(34)
A particle is confined to a one-dimensional box (an infinite well)on the x-axis between x = 0 and x = L.The potential height of the walls of the box is infinite.The normalized wave function of the particle,which is in the ground state,is given by ψ(x)= 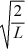 sin
sin 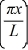 ,with 0 ≤ x ≤ L.What is the maximum probability per unit length of finding the particle?
,with 0 ≤ x ≤ L.What is the maximum probability per unit length of finding the particle?
(Multiple Choice)
4.8/5  (39)
(39)
The square of the wave function of a particle,|ψ(x)|2,gives the probability of finding the particle at the point x.
(True/False)
4.9/5  (24)
(24)
If the accuracy in measuring the position of a particle increases,the accuracy in measuring its velocity will
(Multiple Choice)
4.8/5  (37)
(37)
The wave function for an electron that is confined to x ≥ 0 nm is
ψ(x)= 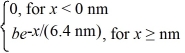 a)What must be the value of b?
(b)What is the probability of finding the electron in a 0.010 nm-wide region centered at x = 1.0 nm?
a)What must be the value of b?
(b)What is the probability of finding the electron in a 0.010 nm-wide region centered at x = 1.0 nm?
(Essay)
4.8/5  (33)
(33)
A molecule of roughly spherical shape has a mass of 6.10 × 10-25 kg and a diameter of 0.70 nm.The uncertainty in the measured position of the molecule is equal to the molecular diameter.What is the minimum uncertainty in the speed of this molecule? (h = 6.626 × 10-34 J ∙ s)
(Multiple Choice)
4.9/5  (23)
(23)
If the accuracy in measuring the velocity of a particle increases,the accuracy in measuring its position will
(Multiple Choice)
4.9/5  (38)
(38)
A nonrelativistic proton is confined to a length of 2.0 pm on the x-axis.What is the kinetic energy of the proton if its speed is equal to the minimum uncertainty possible in its speed? (1 eV = 1.60 × 10-19 J,h = 6.626 × 10-34 J ∙ s,mproton = 1.67 × 10-27 kg)
(Multiple Choice)
4.9/5  (37)
(37)
The wave function for a particle must be normalizable because
(Multiple Choice)
4.9/5  (45)
(45)
An electron inside a hydrogen atom is confined to within a space of 0.110 nm.What is the minimum uncertainty in the electron's velocity? (h = 6.626 × 10-34 J ∙ s,mel = 9.11 × 10-31 kg)
(Multiple Choice)
4.7/5  (33)
(33)
The probability density for an electron that has passed through an experimental apparatus is shown in the figure.If 4100 electrons pass through the apparatus,what is the expected number that will land in a 0.10 mm-wide strip centered at x = 0.00 mm? 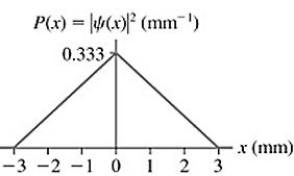
(Multiple Choice)
4.9/5  (35)
(35)
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)