Deck 2: Atoms and the Periodic Table
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/95
Play
Full screen (f)
Deck 2: Atoms and the Periodic Table
1
The active ingredient in the drug Fosamax is a compound with the chemical formula C4H18NNaO10P2. Which statement concerning the chemical formula of this compound is FALSE?
A)Atoms of six different elements make up this compound.
B)Carbon,hydrogen,nitrogen,sodium,oxygen,and potassium atoms are present in this compound.
C)The ratio of carbon atoms to oxygen atoms is 4:10.
D)There is only one atom of nitrogen present in this compound.
A)Atoms of six different elements make up this compound.
B)Carbon,hydrogen,nitrogen,sodium,oxygen,and potassium atoms are present in this compound.
C)The ratio of carbon atoms to oxygen atoms is 4:10.
D)There is only one atom of nitrogen present in this compound.
Carbon,hydrogen,nitrogen,sodium,oxygen,and potassium atoms are present in this compound.
2
Which element is a nonmetal?
A)K
B)Co
C)Br
D)Al
A)K
B)Co
C)Br
D)Al
Br
3
Which of the following properly represents the order of orbital filling based on the relative energy of the orbitals?
A)1s,2s,2p,3s,3p,3d,4s,4p
B)1s,2s,3s,4s,2p,3p,4p,3d
C)1s,2s,2p,3s,3p,4s,3d,4p
D)1s,2s,2p,3s,3d,3p,4s,4p
A)1s,2s,2p,3s,3p,3d,4s,4p
B)1s,2s,3s,4s,2p,3p,4p,3d
C)1s,2s,2p,3s,3p,4s,3d,4p
D)1s,2s,2p,3s,3d,3p,4s,4p
1s,2s,2p,3s,3p,4s,3d,4p
4
The chemical reactivity of an element is determined by which of the following?
A)The number of protons in an atom of the element
B)The number of valence electrons in an atom of the element
C)The number of neutrons in an atom of the element
D)The number of protons and neutrons in an atom of the element
A)The number of protons in an atom of the element
B)The number of valence electrons in an atom of the element
C)The number of neutrons in an atom of the element
D)The number of protons and neutrons in an atom of the element

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
5
Which atom has the smallest atomic radius?
A)K
B)Ga
C)Br
D)Rb
A)K
B)Ga
C)Br
D)Rb

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
6
Which element is not an alkali metal?
A)Li
B)K
C)Rb
D)H
E)All of these elements are alkali metals.
A)Li
B)K
C)Rb
D)H
E)All of these elements are alkali metals.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
7
Which element is a metalloid?
A)B
B)C
C)Ar
D)Al
A)B
B)C
C)Ar
D)Al

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
8
Which element is a metal?
A)Li
B)Si
C)Cl
D)Ar
E)More than one of the elements is a metal.
A)Li
B)Si
C)Cl
D)Ar
E)More than one of the elements is a metal.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
9
What is the mass number of the isotope with the symbol 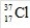 ?
?
A)17
B)18
C)35.45
D)37
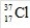 ?
?A)17
B)18
C)35.45
D)37

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
10
Which statement is not part of the modern description of the electronic structure of an atom?
A)Electrons occupy discrete energy levels.
B)Electrons move freely in space.
C)The energy of electrons is quantized.
D)The energy of electrons is restricted to specific values.
A)Electrons occupy discrete energy levels.
B)Electrons move freely in space.
C)The energy of electrons is quantized.
D)The energy of electrons is restricted to specific values.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
11
How many protons are in the isotope with the symbol 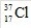 ?
?
A)17
B)18
C)35.45
D)37
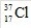 ?
?A)17
B)18
C)35.45
D)37

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
12
Which element is not an alkali metal?
A)Li
B)Kr
C)Rb
D)Na
E)All of these elements are alkali metals.
A)Li
B)Kr
C)Rb
D)Na
E)All of these elements are alkali metals.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
13
The element symbol for manganese is ________.
A)M
B)Ma
C)Mg
D)Mn
A)M
B)Ma
C)Mg
D)Mn

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
14
The element symbol for sulfur is ________.
A)S
B)Su
C)Sf
D)Sl
A)S
B)Su
C)Sf
D)Sl

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
15
Which element is a noble gas?
A)H
B)Ne
C)Pr
D)Ra
E)More than one of the elements listed is a noble gas.
A)H
B)Ne
C)Pr
D)Ra
E)More than one of the elements listed is a noble gas.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
16
Silicon has three naturally occurring isotopes: Si-28,Si-29,and Si-30. If the average atomic mass of silicon is 28.09,which isotope has the highest isotopic abundance?
A)Si-28
B)Si-29
C)Si-30
D)All isotopes have the same isotopic abundance.
A)Si-28
B)Si-29
C)Si-30
D)All isotopes have the same isotopic abundance.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
17
Which element is a transition metal in period 4?
A)K
B)Hf
C)Sn
D)Sc
A)K
B)Hf
C)Sn
D)Sc

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
18
What is the atomic number of the isotope with the symbol 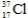 ?
?
A)17
B)18
C)35.45
D)37
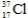 ?
?A)17
B)18
C)35.45
D)37

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
19
What is the maximum number of electrons that can occupy the third (n=3)shell?
A)2
B)3
C)6
D)8
E)18
A)2
B)3
C)6
D)8
E)18

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
20
Which atom has the largest atomic radius?
A)K
B)Ga
C)Br
D)Rb
A)K
B)Ga
C)Br
D)Rb

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
21
The proper electron-dot symbol for aluminum is ________.
A)
B)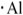
C)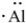
D)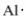
A)

B)
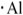
C)
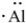
D)
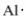

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
22
Which element is chemically similar to lithium?
A)Sulfur
B)Magnesium
C)Iron
D)Lanthanum
E)Potassium
A)Sulfur
B)Magnesium
C)Iron
D)Lanthanum
E)Potassium

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
23
Which element is an s block element?
A)S
B)Ar
C)He
D)La
E)None of these elements is an s block element.
A)S
B)Ar
C)He
D)La
E)None of these elements is an s block element.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
24
Which element has the following orbital diagram? 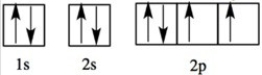
A)O
B)N
C)C
D)Li

A)O
B)N
C)C
D)Li

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
25
Which element has the following orbital diagram? 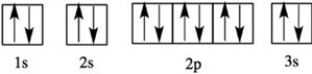
A)Li
B)Be
C)Na
D)Mg

A)Li
B)Be
C)Na
D)Mg

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
26
Which element is chemically similar to chlorine?
A)Sulfur
B)Calcium
C)Oxygen
D)Bromine
E)Argon
A)Sulfur
B)Calcium
C)Oxygen
D)Bromine
E)Argon

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
27
In the diagram below,which highlighted element is an f block element? 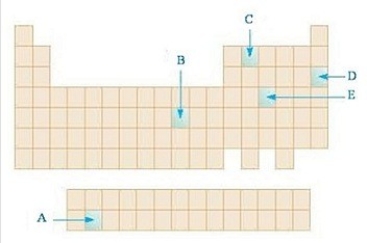
A)A
B)B
C)C
D)D
E)E

A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
28
How many neutrons are in the isotope 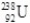 ?
?
A)238
B)146
C)92
D)330
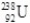 ?
?A)238
B)146
C)92
D)330

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
29
Which element is most likely to be a good conductor of electricity?
A)Ar
B)N
C)F
D)Ni
E)O
A)Ar
B)N
C)F
D)Ni
E)O

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
30
Which element has the smallest ionization energy?
A)K
B)Ga
C)Br
D)Rb
A)K
B)Ga
C)Br
D)Rb

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
31
Which element has the following orbital diagram? 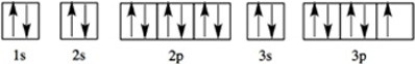
A)Ar
B)Cl
C)F
D)S

A)Ar
B)Cl
C)F
D)S

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
32
The elements in a column of the periodic table are collectively referred to as ________.
A)metals
B)a period
C)a group
D)a series
E)metalloids
A)metals
B)a period
C)a group
D)a series
E)metalloids

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
33
An atom of the isotope chlorine-37 consists of how many protons,neutrons,and electrons? (p = proton,n = neutron,e = electron)
A)18 p,37 n,18 e
B)17 p,20 n,17 e
C)17 p,20 n,18 e
D)37 p,37 n,17 e
E)37 p,20 n,37 e
A)18 p,37 n,18 e
B)17 p,20 n,17 e
C)17 p,20 n,18 e
D)37 p,37 n,17 e
E)37 p,20 n,37 e

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
34
Which isotope is not possible?
A)
B)
C)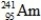
D)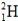
E)More than one of the isotopes is not possible.
A)
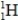
B)
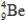
C)
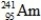
D)
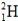
E)More than one of the isotopes is not possible.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
35
How many electrons are in the isotope 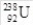 ?
?
A)238
B)146
C)92
D)330
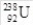 ?
?A)238
B)146
C)92
D)330

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
36
Which element is a d block element?
A)S
B)Ar
C)Ag
D)As
E)None of these elements is a d block element.
A)S
B)Ar
C)Ag
D)As
E)None of these elements is a d block element.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
37
What is the symbol for the isotope with A = 31 and Z = 15?
A)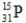
B)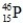
C)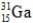
D)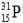
A)
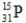
B)
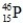
C)
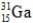
D)
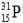

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
38
The electron configuration of chlorine is 1s22s22p63s23p5. Which statement about chlorine is incorrect?
A)Chlorine has five valence electrons
B)Chlorine's valence shell is the third shell
C)Chlorine has five electrons in the 3p subshell
D)Chlorine has 17 total electrons
A)Chlorine has five valence electrons
B)Chlorine's valence shell is the third shell
C)Chlorine has five electrons in the 3p subshell
D)Chlorine has 17 total electrons

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
39
Which element has the following orbital diagram? 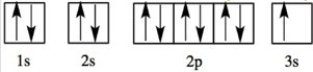
A)Mg
B)K
C)Na
D)S

A)Mg
B)K
C)Na
D)S

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
40
How many protons are in the isotope 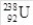 ?
?
A)238
B)146
C)92
D)330
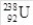 ?
?A)238
B)146
C)92
D)330

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
41
Which element is located in period 3,Group 3A?
A)Al
B)S
C)Si
D)B
A)Al
B)S
C)Si
D)B

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
42
The element symbol S represents sodium.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
43
Helium is an s block element.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
44
The nucleus contains most of the mass of an atom and is positively charged.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
45
Electrons are negatively charged and have the smallest mass of the three subatomic particles.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
46
Antimony is a metalloid containing 51 protons that is alloyed with lead and used in car batteries. What is the element symbol for antimony?
A)A
B)An
C)At
D)Sb
E)Cr
A)A
B)An
C)At
D)Sb
E)Cr

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
47
Nonmetals have a shiny appearance,and they are generally poor conductors of heat and electricity.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
48
What is the Period and Group for the element Bromine?
A)Period 4,Group 7A
B)Period 7,Group 4A
C)Period 2,Group 3A
D)Period 6,Group 2A
A)Period 4,Group 7A
B)Period 7,Group 4A
C)Period 2,Group 3A
D)Period 6,Group 2A

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
49
Zirconium (Zr)is an element classified as a metal. Which property cannot be assumed based on its classification as a metal?
A)Zr has a relatively high density
B)Zr is a trace element in the body
C)Zr is a good conductor of electricity
D)Zr is a shiny solid
A)Zr has a relatively high density
B)Zr is a trace element in the body
C)Zr is a good conductor of electricity
D)Zr is a shiny solid

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
50
Hydrogen is located in group 1A but it is not considered an alkali metal.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
51
Fl is the element symbol for fluorine.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
52
What is the period and Group for the element Boron?
A)Period 2,Group 13A
B)Period 3,Group 3A
C)Period 2,Group 3A
D)Period 4,Group 8A
A)Period 2,Group 13A
B)Period 3,Group 3A
C)Period 2,Group 3A
D)Period 4,Group 8A

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
53
An alloy is a mixture of two or more elements that has metallic properties.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
54
The element symbol for iron is Fe.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
55
All elements have at least two naturally occurring isotopes.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
56
A sulfur atom has a larger atomic radius than an oxygen atom. Which statement best explains why?
A)Sulfur contains more electrons than oxygen does.
B)Sulfur contains more protons than oxygen does.
C)The valence shell of sulfur is farther away from the nucleus than the valence shell of oxygen is.
D)The larger number of protons in an oxygen atom pulls its electrons closer to the nucleus than a sulfur atom.
A)Sulfur contains more electrons than oxygen does.
B)Sulfur contains more protons than oxygen does.
C)The valence shell of sulfur is farther away from the nucleus than the valence shell of oxygen is.
D)The larger number of protons in an oxygen atom pulls its electrons closer to the nucleus than a sulfur atom.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
57
All atoms of the same element contain the same number of protons.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
58
Which statement concerning the elements fluorine,chlorine,bromine,and iodine is INCORRECT?
A)These elements are all halogens.
B)These elements all have the same valence shell.
C)These elements are all nonmetals.
D)These elements all have the same number of valence electrons.
A)These elements are all halogens.
B)These elements all have the same valence shell.
C)These elements are all nonmetals.
D)These elements all have the same number of valence electrons.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
59
Protons and electrons reside in the nucleus of an atom.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
60
Which statement describing atoms is FALSE?
A)The number of protons in an atom is referred to as the atomic number of the atom.
B)The total number of protons,neutrons,and electrons in an atom is referred to as the mass number of the atom.
C)Protons and neutrons are located in the nucleus of an atom.
D)Electrons are located in the space outside the nucleus called the electron cloud.
A)The number of protons in an atom is referred to as the atomic number of the atom.
B)The total number of protons,neutrons,and electrons in an atom is referred to as the mass number of the atom.
C)Protons and neutrons are located in the nucleus of an atom.
D)Electrons are located in the space outside the nucleus called the electron cloud.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
61
The maximum number of electrons that can occupy the 3d subshell is ten (10).

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
62
Sulfur is a metal.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
63
The farther a shell is from the nucleus,the larger its volume becomes,and the more electrons it can hold.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
64
Oxygen,carbon,hydrogen,and nitrogen are called the building-block elements because they make up the majority of the mass of the human body.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
65
Iodine has smaller ionization energy than chlorine.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
66
The electron-dot symbol for barium is 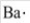 .
.
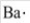 .
.
Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
67
A compound is a pure substance formed by chemically combining two or more elements together.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
68
Group 6A elements have the general electron configuration of ns2np6.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
69
The electron configuration for calcium is 1s22s22p63s23p64s2.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
70
When orbitals are equal in energy,one electron is added to each orbital until the orbitals are half-filled,before any orbital is completely filled.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
71
Phosphorus has 15 valence electrons.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
72
The 5s orbital is lower in energy than the 4d orbital.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
73
When two electrons occupy the same orbital they have paired spins-that is,the spins are opposite in direction.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
74
The electron cloud contains most of the volume of an atom.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
75
Magnesium is a metal.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
76
A column in the periodic table is called a period.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
77
The mass of a neutron is equal to the mass of a proton plus the mass of an electron.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
78
A bromine atom is smaller than a potassium atom.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
79
Bromine is abbreviated by the two-letter symbol BR.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
80
All metals are solids at room temperature.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck


