Exam 2: Atoms and the Periodic Table
Exam 1: Matter and Measurement87 Questions
Exam 2: Atoms and the Periodic Table95 Questions
Exam 3: Ionic Compounds100 Questions
Exam 4: Covalent Compounds101 Questions
Exam 5: Chemical Reactions98 Questions
Exam 6: Energy Changes,reaction Rates,and Equilibrium102 Questions
Exam 7: Gases,liquids,and Solids98 Questions
Exam 8: Solutions98 Questions
Exam 9: Acids and Bases108 Questions
Exam 10: Nuclear Chemistry93 Questions
Exam 11: Introduction to Organic Molecules and Functional Groups123 Questions
Exam 12: Alkanes104 Questions
Exam 13: Unsaturated Hydrocarbons104 Questions
Exam 14: Organic Compounds That Contain Oxygen,halogen,or Sulfur112 Questions
Exam 15: The Three-Dimensional Shape of Molecules101 Questions
Exam 16: Aldehydes and Ketones114 Questions
Exam 17: Carboxylic Acids,esters,and Amides107 Questions
Exam 18: Amines and Neurotransmitters115 Questions
Exam 19: Lipids115 Questions
Exam 20: Carbohydrates100 Questions
Exam 21: Amino Acids,proteins,and Enzymes98 Questions
Exam 22: Nucleic Acids and Protein Synthesis98 Questions
Exam 23: Metabolism and Energy Production102 Questions
Exam 24: Carbohydrate,lipid,and Protein Metabolism99 Questions
Select questions type
The proper electron-dot symbol for aluminum is ________.
Free
(Multiple Choice)
4.8/5  (30)
(30)
Correct Answer:
A
What is the Period and Group for the element Bromine?
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
A
What is the mass number of the isotope with the symbol 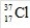 ?
?
Free
(Multiple Choice)
4.8/5  (30)
(30)
Correct Answer:
D
Nonmetals have a shiny appearance,and they are generally poor conductors of heat and electricity.
(True/False)
4.9/5  (27)
(27)
An alloy is a mixture of two or more elements that has metallic properties.
(True/False)
4.9/5  (39)
(39)
Isotopes of the same element have the same number of ________.
(Short Answer)
4.9/5  (38)
(38)
Group 6A elements have the general electron configuration of ns2np6.
(True/False)
4.9/5  (31)
(31)
Oxygen,carbon,hydrogen,and nitrogen are called the building-block elements because they make up the majority of the mass of the human body.
(True/False)
4.7/5  (31)
(31)
An atom with A = 21 and Z = 10 is an isotope of an atom with A = 20 and Z = 10.
(True/False)
4.8/5  (34)
(34)
The nucleus contains most of the mass of an atom and is positively charged.
(True/False)
4.9/5  (37)
(37)
Which of the following properly represents the order of orbital filling based on the relative energy of the orbitals?
(Multiple Choice)
4.7/5  (35)
(35)
Showing 1 - 20 of 95
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)