Deck 27: Early Quantum Theory and Models of the Atom
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/192
Play
Full screen (f)
Deck 27: Early Quantum Theory and Models of the Atom
1
Choose the one alternative that best completes the statement or answers the question.
A blue laser beam is incident on a metallic surface, causing electrons to be ejected from the metal. If the frequency of the laser beam is increased while the intensity of the beam is held fixed,
A) the rate of ejected electrons will remain the same but their maximum kinetic energy will increase.
B) the rate of ejected electrons will remain the same but their maximum kinetic energy will decrease.
C) the rate of ejected electrons will decrease and their maximum kinetic energy will increase.
D) the rate of ejected electrons will increase and their maximum kinetic energy will increase.
A blue laser beam is incident on a metallic surface, causing electrons to be ejected from the metal. If the frequency of the laser beam is increased while the intensity of the beam is held fixed,
A) the rate of ejected electrons will remain the same but their maximum kinetic energy will increase.
B) the rate of ejected electrons will remain the same but their maximum kinetic energy will decrease.
C) the rate of ejected electrons will decrease and their maximum kinetic energy will increase.
D) the rate of ejected electrons will increase and their maximum kinetic energy will increase.
C
2
Choose the one alternative that best completes the statement or answers the question.
Two sources emit beams of microwaves. The microwaves from source A have a frequency of 10 GHz, and the ones from source B have a frequency of 20 GHz. This is all we know about the two
Beams. Which of the following statements about these beams are correct? (There could be more
Than one correct choice.)
A) The intensity of beam B is twice as great as the intensity of beam A.
B) A photon in beam B has the same energy as a photon in beam A.
C) A photon in beam B has twice the energy of a photon in beam A.
D) Beam B carries twice as many photons per second as beam A.
E) None of the above statements are true.
Two sources emit beams of microwaves. The microwaves from source A have a frequency of 10 GHz, and the ones from source B have a frequency of 20 GHz. This is all we know about the two
Beams. Which of the following statements about these beams are correct? (There could be more
Than one correct choice.)
A) The intensity of beam B is twice as great as the intensity of beam A.
B) A photon in beam B has the same energy as a photon in beam A.
C) A photon in beam B has twice the energy of a photon in beam A.
D) Beam B carries twice as many photons per second as beam A.
E) None of the above statements are true.
C
3
Choose the one alternative that best completes the statement or answers the question.
A beam of light falling on a metal surface is causing electrons to be ejected from the surface. If we now double the frequency of the light, which of the following statements are correct? (There could
Be more than one correct choice.)
A) The number of electrons ejected per second doubles.
B) The speed of the ejected electrons doubles.
C) Twice as many photons hit the metal surface as before.
D) The kinetic energy of the ejected electrons doubles.
E) None of the above things occur.
A beam of light falling on a metal surface is causing electrons to be ejected from the surface. If we now double the frequency of the light, which of the following statements are correct? (There could
Be more than one correct choice.)
A) The number of electrons ejected per second doubles.
B) The speed of the ejected electrons doubles.
C) Twice as many photons hit the metal surface as before.
D) The kinetic energy of the ejected electrons doubles.
E) None of the above things occur.
E
4
Choose the one alternative that best completes the statement or answers the question.
A photon of blue light and a photon of red light are traveling in vacuum. The photon of blue light
A) has a longer wavelength than a photon of red light and travels with the same speed.
B) has a longer wavelength than a photon of red light and travels with a greater speed.
C) has a smaller wavelength than a photon of red light and travels with the same speed.
D) has a smaller wavelength than a photon of red light and travels with a greater speed.
A photon of blue light and a photon of red light are traveling in vacuum. The photon of blue light
A) has a longer wavelength than a photon of red light and travels with the same speed.
B) has a longer wavelength than a photon of red light and travels with a greater speed.
C) has a smaller wavelength than a photon of red light and travels with the same speed.
D) has a smaller wavelength than a photon of red light and travels with a greater speed.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
5
Choose the one alternative that best completes the statement or answers the question.
Photon A has twice the momentum of photon B as both of them are traveling in vacuum. Which statements about these photons are correct? (There could be more than one correct choice.)
A) Photon A is traveling twice as fast as photon B.
B) Both photons have the same speed.
C) The wavelength of photon A is twice as great as the wavelength of photon B.
D) Both photons have the same wavelength.
E) The wavelength of photon B is twice as great as the wavelength of photon A.
Photon A has twice the momentum of photon B as both of them are traveling in vacuum. Which statements about these photons are correct? (There could be more than one correct choice.)
A) Photon A is traveling twice as fast as photon B.
B) Both photons have the same speed.
C) The wavelength of photon A is twice as great as the wavelength of photon B.
D) Both photons have the same wavelength.
E) The wavelength of photon B is twice as great as the wavelength of photon A.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
6
Choose the one alternative that best completes the statement or answers the question.
If the wavelength of a photon is the same as the de Broglie wavelength of an electron, which one has the greater momentum?
A) The photon because it is traveling faster.
B) The electron because it has more mass.
C) They both have the same momentum.
If the wavelength of a photon is the same as the de Broglie wavelength of an electron, which one has the greater momentum?
A) The photon because it is traveling faster.
B) The electron because it has more mass.
C) They both have the same momentum.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
7
Choose the one alternative that best completes the statement or answers the question.
If the frequency of a light beam is doubled, what happens to the momentum of the photons in that beam of light?
A) It is reduced to one-fourth of its original value.
B) It is increased to four times its original value.
C) It stays the same.
D) It is halved.
E) It is doubled.
If the frequency of a light beam is doubled, what happens to the momentum of the photons in that beam of light?
A) It is reduced to one-fourth of its original value.
B) It is increased to four times its original value.
C) It stays the same.
D) It is halved.
E) It is doubled.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
8
Choose the one alternative that best completes the statement or answers the question.
If the wavelength of a light beam is doubled, what happens to the momentum of the photons in that light beam?
A) It stays the same.
B) It is reduced by one-fourth of its original value.
C) It is halved.
D) It is doubled.
E) It is increased to four times its original value.
If the wavelength of a light beam is doubled, what happens to the momentum of the photons in that light beam?
A) It stays the same.
B) It is reduced by one-fourth of its original value.
C) It is halved.
D) It is doubled.
E) It is increased to four times its original value.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
9
Choose the one alternative that best completes the statement or answers the question.
Monochromatic light falls on a metal surface and electrons are ejected. If the intensity of the light is increased, what will happen to the ejection rate and maximum energy of the electrons?
A) greater rate; greater maximum energy.
B) same rate; same maximum energy.
C) same rate; greater maximum energy.
D) greater rate; same maximum energy.
Monochromatic light falls on a metal surface and electrons are ejected. If the intensity of the light is increased, what will happen to the ejection rate and maximum energy of the electrons?
A) greater rate; greater maximum energy.
B) same rate; same maximum energy.
C) same rate; greater maximum energy.
D) greater rate; same maximum energy.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
10
Choose the one alternative that best completes the statement or answers the question.
Which of the following actions will increase the energy of a photon? (There could be more than one correct choice.)
A) Decrease its frequency.
B) Increase its speed.
C) Increase its wavelength.
D) Decrease its wavelength.
E) Increase its frequency.
Which of the following actions will increase the energy of a photon? (There could be more than one correct choice.)
A) Decrease its frequency.
B) Increase its speed.
C) Increase its wavelength.
D) Decrease its wavelength.
E) Increase its frequency.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
11
Choose the one alternative that best completes the statement or answers the question.
Increasing the brightness of a beam of light without changing its color will increase
A) the number of photons per second traveling in the beam.
B) the wavelength of the photons.
C) the energy of each photon.
D) the frequency of the light.
E) the speed of the photons.
Increasing the brightness of a beam of light without changing its color will increase
A) the number of photons per second traveling in the beam.
B) the wavelength of the photons.
C) the energy of each photon.
D) the frequency of the light.
E) the speed of the photons.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
12
Choose the one alternative that best completes the statement or answers the question.
As the temperature of a blackbody increases, what happens to the peak wavelength of the light it radiates?
A) It gets shorter.
B) It gets longer.
C) The wavelength is not affected by the temperature of the object.
As the temperature of a blackbody increases, what happens to the peak wavelength of the light it radiates?
A) It gets shorter.
B) It gets longer.
C) The wavelength is not affected by the temperature of the object.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
13
Choose the one alternative that best completes the statement or answers the question.
If you double the frequency of the light in a laser beam, but keep the number of photons per second in the beam fixed, which of the following statements are correct? (There could be more
Than one correct choice.)
A) The energy of individual photons doubles.
B) The power in the beam does not change.
C) The intensity of the beam doubles.
D) The energy of individual photons does not change.
E) The wavelength of the individual photons doubles.
If you double the frequency of the light in a laser beam, but keep the number of photons per second in the beam fixed, which of the following statements are correct? (There could be more
Than one correct choice.)
A) The energy of individual photons doubles.
B) The power in the beam does not change.
C) The intensity of the beam doubles.
D) The energy of individual photons does not change.
E) The wavelength of the individual photons doubles.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
14
Choose the one alternative that best completes the statement or answers the question.
When the surface of a metal is exposed to blue light, electrons are emitted. If the intensity of the blue light is increased, which of the following things will also increase?
A) the time lag between the onset of the absorption of light and the ejection of electrons
B) the work function of the metal
C) the number of electrons ejected per second
D) the maximum kinetic energy of the ejected electrons
E) all of the above
When the surface of a metal is exposed to blue light, electrons are emitted. If the intensity of the blue light is increased, which of the following things will also increase?
A) the time lag between the onset of the absorption of light and the ejection of electrons
B) the work function of the metal
C) the number of electrons ejected per second
D) the maximum kinetic energy of the ejected electrons
E) all of the above

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
15
Choose the one alternative that best completes the statement or answers the question.
A photon scatters off of a stationary electron. Which of the following statements about the photon are true? (There could be more than one correct choice.)
A) Its energy does not change.
B) Its wavelength increases due to the scattering.
C) Its frequency increases due to the scattering.
D) Its frequency decreases due to the scattering.
E) Its wavelength decreases due to the scattering.
A photon scatters off of a stationary electron. Which of the following statements about the photon are true? (There could be more than one correct choice.)
A) Its energy does not change.
B) Its wavelength increases due to the scattering.
C) Its frequency increases due to the scattering.
D) Its frequency decreases due to the scattering.
E) Its wavelength decreases due to the scattering.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
16
Choose the one alternative that best completes the statement or answers the question.
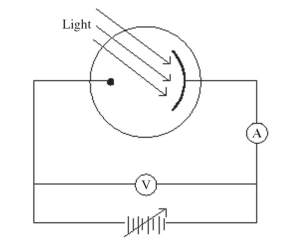
Monochromatic light is incident on a metal surface, and the ejected electrons give rise to a current in the circuit shown in the figure. The maximum kinetic energy of the ejected electrons is determined by applying a reverse ('stopping') potential, sufficient to reduce the current in the ammeter to zero. If the intensity of the incident light is increased, how will the required stopping potential change?
A) It will increase.
B) It will decrease.
C) It will remain unchanged.
Monochromatic light is incident on a metal surface, and the ejected electrons give rise to a current in the circuit shown in the figure. The maximum kinetic energy of the ejected electrons is determined by applying a reverse ('stopping') potential, sufficient to reduce the current in the ammeter to zero. If the intensity of the incident light is increased, how will the required stopping potential change?

A) It will increase.
B) It will decrease.
C) It will remain unchanged.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
17
Choose the one alternative that best completes the statement or answers the question.
Two identical metal bars are heated up until they are both glowing. One of them is "red hot" and the other is "blue hot." Which one is hotter, the one that glows red or the one that glows blue?
A) the red one
B) the blue one
C) We cannot tell without knowing more about the two bars.
Two identical metal bars are heated up until they are both glowing. One of them is "red hot" and the other is "blue hot." Which one is hotter, the one that glows red or the one that glows blue?
A) the red one
B) the blue one
C) We cannot tell without knowing more about the two bars.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
18
Choose the one alternative that best completes the statement or answers the question.
Light of a given wavelength is used to illuminate the surface of a metal, however, no photoelectrons are emitted. In order to cause electrons to be ejected from the surface of this metal
You should
A) use light of the same wavelength but increase its intensity.
B) use light of the same wavelength but decrease its intensity.
C) use light of a longer wavelength.
D) use light of a shorter wavelength.
Light of a given wavelength is used to illuminate the surface of a metal, however, no photoelectrons are emitted. In order to cause electrons to be ejected from the surface of this metal
You should
A) use light of the same wavelength but increase its intensity.
B) use light of the same wavelength but decrease its intensity.
C) use light of a longer wavelength.
D) use light of a shorter wavelength.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
19
Choose the one alternative that best completes the statement or answers the question.
If the wavelength of a photon is doubled, what happens to its energy?
A) It stays the same.
B) It is increased to four times its original value.
C) It is doubled.
D) It is reduced to one-fourth of its original value.
E) It is reduced to one-half of its original value.
If the wavelength of a photon is doubled, what happens to its energy?
A) It stays the same.
B) It is increased to four times its original value.
C) It is doubled.
D) It is reduced to one-fourth of its original value.
E) It is reduced to one-half of its original value.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
20
Choose the one alternative that best completes the statement or answers the question.
Two sources emit beams of light of wavelength . The light from source A has an intensity of , and the light from source has an intensity of . This is all we know about the two beams. Which of the following statements about these beams are correct? (There could be more than one correct choice.)
A) A photon in beam B has the same energy as a photon in beam A.
B) photon in beam has twice the energy of a photon in beam .
C) The frequency of the light in beam is twice as great as the frequency of the light in beam .
D) Beam B carries twice as many photons per second as beam A.
E) None of the above statements are true.
Two sources emit beams of light of wavelength . The light from source A has an intensity of , and the light from source has an intensity of . This is all we know about the two beams. Which of the following statements about these beams are correct? (There could be more than one correct choice.)
A) A photon in beam B has the same energy as a photon in beam A.
B) photon in beam has twice the energy of a photon in beam .
C) The frequency of the light in beam is twice as great as the frequency of the light in beam .
D) Beam B carries twice as many photons per second as beam A.
E) None of the above statements are true.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
21
Choose the one alternative that best completes the statement or answers the question.
A proton and an electron are both accelerated to the same final speed. If is the de Broglie wavelength of the proton and is the de Broglie wavelength of the electron, then
A) .
B)
C) .
A proton and an electron are both accelerated to the same final speed. If is the de Broglie wavelength of the proton and is the de Broglie wavelength of the electron, then
A) .
B)
C) .

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
22
Choose the one alternative that best completes the statement or answers the question.
If the wavelength of a photon in vacuum is the same as the de Broglie wavelength of an electron, which one is traveling faster through space?
A) The electron because it has more mass.
B) The photon because photons always travel through space faster than electrons.
C) They both have the same speed.
If the wavelength of a photon in vacuum is the same as the de Broglie wavelength of an electron, which one is traveling faster through space?
A) The electron because it has more mass.
B) The photon because photons always travel through space faster than electrons.
C) They both have the same speed.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
23
Choose the one alternative that best completes the statement or answers the question.
To which of the following values of does the longest wavelength in the Balmer series correspond?
A) 5
B) 3
C) 1
D) 7
E) (very large)
To which of the following values of does the longest wavelength in the Balmer series correspond?
A) 5
B) 3
C) 1
D) 7
E) (very large)

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
24
Choose the one alternative that best completes the statement or answers the question.
The Paschen series is formed by electron transitions that
A) end on the n = 3 shell.
B) begin on the n = 1 shell.
C) end on the n = 2 shell.
D) begin on the n = 3 shell.
E) end on the n = 1 shell.
The Paschen series is formed by electron transitions that
A) end on the n = 3 shell.
B) begin on the n = 1 shell.
C) end on the n = 2 shell.
D) begin on the n = 3 shell.
E) end on the n = 1 shell.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
25
Choose the one alternative that best completes the statement or answers the question.
The figure shows part of the energy level diagram of a certain atom. The energy spacing between levels 1 and 2 is twice that between 2 and 3 . If an electron makes a transition from level 3 to level 2 , the radiation of wavelength is emitted. What possible radiation wavelengths might be produced by other transitions between the three energy levels?
A) only
B) both and
C) only
D) both and
The figure shows part of the energy level diagram of a certain atom. The energy spacing between levels 1 and 2 is twice that between 2 and 3 . If an electron makes a transition from level 3 to level 2 , the radiation of wavelength is emitted. What possible radiation wavelengths might be produced by other transitions between the three energy levels?
A) only
B) both and
C) only
D) both and

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
26
Choose the one alternative that best completes the statement or answers the question.
Hydrogen atoms can emit four spectral lines with visible colors from red to violet. These four visible lines emitted by hydrogen atoms are produced by electrons
A) that end up in the n = 3 level.
B) that start in the ground state.
C) that end up in the ground state.
D) that end up in the n = 2 level.
E) that start in the n = 2 level.
Hydrogen atoms can emit four spectral lines with visible colors from red to violet. These four visible lines emitted by hydrogen atoms are produced by electrons
A) that end up in the n = 3 level.
B) that start in the ground state.
C) that end up in the ground state.
D) that end up in the n = 2 level.
E) that start in the n = 2 level.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
27
Choose the one alternative that best completes the statement or answers the question.
Protons are being accelerated in a particle accelerator. When the speed of the protons is doubled, their de Broglie wavelength will
A) decrease by a factor of .
B) increase by a factor of 4 .
C) decrease by a factor of 2 .
D) increase by a factor of .
E) increase by a factor of 2 .
Protons are being accelerated in a particle accelerator. When the speed of the protons is doubled, their de Broglie wavelength will
A) decrease by a factor of .
B) increase by a factor of 4 .
C) decrease by a factor of 2 .
D) increase by a factor of .
E) increase by a factor of 2 .

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
28
Choose the one alternative that best completes the statement or answers the question.
If a proton and an electron have the same de Broglie wavelengths, which one is moving faster?
A) the proton
B) the electron
C) They both have the same speed.
If a proton and an electron have the same de Broglie wavelengths, which one is moving faster?
A) the proton
B) the electron
C) They both have the same speed.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
29
Choose the one alternative that best completes the statement or answers the question.
A proton and an electron are both accelerated to the same final kinetic energy. If is the de Broglie wavelength of the proton and is the de Broglie wavelength of the electron, then
A) .
B) .
C) .
A proton and an electron are both accelerated to the same final kinetic energy. If is the de Broglie wavelength of the proton and is the de Broglie wavelength of the electron, then
A) .
B) .
C) .

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
30
Choose the one alternative that best completes the statement or answers the question.
When an electron jumps from an orbit where n = 4 to one where n = 2
A) a photon is emitted.
B) two photons are absorbed.
C) a photon is absorbed.
D) two photons are emitted.
E) None of the given answers are correct.
When an electron jumps from an orbit where n = 4 to one where n = 2
A) a photon is emitted.
B) two photons are absorbed.
C) a photon is absorbed.
D) two photons are emitted.
E) None of the given answers are correct.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
31
Choose the one alternative that best completes the statement or answers the question.
The distance between adjacent orbits in a hydrogen atom
A) remains constant for all values of .
B) decreases with increasing values of .
C) varies randomly with increasing values of .
D) increases with increasing values of .
The distance between adjacent orbits in a hydrogen atom
A) remains constant for all values of .
B) decreases with increasing values of .
C) varies randomly with increasing values of .
D) increases with increasing values of .

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
32
Choose the one alternative that best completes the statement or answers the question.
To which of the following values of does the shortest wavelength in the Balmer series correspond?
A) 5
B) 7
C) 3
D) 1
E) (very large)
To which of the following values of does the shortest wavelength in the Balmer series correspond?
A) 5
B) 7
C) 3
D) 1
E) (very large)

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
33
Choose the one alternative that best completes the statement or answers the question.
The Lyman series is formed by electron transitions in hydrogen that
A) begin on the shell.
B) begin on the shell.
C) end on the shell.
D) end on the shell.
E) are between the and shells.
The Lyman series is formed by electron transitions in hydrogen that
A) begin on the shell.
B) begin on the shell.
C) end on the shell.
D) end on the shell.
E) are between the and shells.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
34
Choose the one alternative that best completes the statement or answers the question.
Which of the following actions will increase the de Broglie wavelength of a speck of dust? (There could be more than one correct choice.)
A) Decrease its mass.
B) Decrease its speed.
C) Increase its mass.
D) Increase its speed.
E) Decrease its momentum.
Which of the following actions will increase the de Broglie wavelength of a speck of dust? (There could be more than one correct choice.)
A) Decrease its mass.
B) Decrease its speed.
C) Increase its mass.
D) Increase its speed.
E) Decrease its momentum.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
35
Choose the one alternative that best completes the statement or answers the question.
The energy difference between adjacent orbit radii in a hydrogen atom
A) increases with increasing values of n.
B) remains constant for all values of n.
C) varies randomly with increasing values of n.
D) decreases with increasing values of n.
The energy difference between adjacent orbit radii in a hydrogen atom
A) increases with increasing values of n.
B) remains constant for all values of n.
C) varies randomly with increasing values of n.
D) decreases with increasing values of n.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
36
Choose the one alternative that best completes the statement or answers the question.
If a hydrogen atom originally in a state with principal quantum number n is excited to state n' = 2n, then
A) its radius will quadruple and the binding energy will double.
B) its radius will quadruple and the binding energy will be reduced by a factor of four.
C) its radius and binding energy will quadruple.
D) its radius and binding energy will double.
E) its radius will double and the binding energy will quadruple.
If a hydrogen atom originally in a state with principal quantum number n is excited to state n' = 2n, then
A) its radius will quadruple and the binding energy will double.
B) its radius will quadruple and the binding energy will be reduced by a factor of four.
C) its radius and binding energy will quadruple.
D) its radius and binding energy will double.
E) its radius will double and the binding energy will quadruple.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
37
Choose the one alternative that best completes the statement or answers the question.
An ionized atom having protons has had all but one of its electrons removed. If is the total energy of the ground state electron in atomic hydrogen, then what is the total energy of the remaining electron in the ionized atom?
A)
B)
C)
D)
E)
An ionized atom having protons has had all but one of its electrons removed. If is the total energy of the ground state electron in atomic hydrogen, then what is the total energy of the remaining electron in the ionized atom?
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
38
Choose the one alternative that best completes the statement or answers the question.
The Balmer series is formed by electron transitions in hydrogen that
A) end on the shell.
B) begin on the shell.
C) begin on the shell.
D) end on the shell.
E) are between the and shells.
The Balmer series is formed by electron transitions in hydrogen that
A) end on the shell.
B) begin on the shell.
C) begin on the shell.
D) end on the shell.
E) are between the and shells.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
39
Choose the one alternative that best completes the statement or answers the question.
Protons are being accelerated in a particle accelerator. When the energy of the protons is doubled, their de Broglie wavelength will
A) decrease by a factor of 2 .
B) decrease by a factor of .
C) increase by a factor of 2 .
D) increase by a factor of 4 .
E) increase by a factor of .
Protons are being accelerated in a particle accelerator. When the energy of the protons is doubled, their de Broglie wavelength will
A) decrease by a factor of 2 .
B) decrease by a factor of .
C) increase by a factor of 2 .
D) increase by a factor of 4 .
E) increase by a factor of .

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
40
Choose the one alternative that best completes the statement or answers the question.
If a proton and an electron have the same speed, which one has the longer de Broglie wavelength?
A) the electron
B) the proton
C) It is the same for both of them.
If a proton and an electron have the same speed, which one has the longer de Broglie wavelength?
A) the electron
B) the proton
C) It is the same for both of them.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
41
Write the word or phrase that best completes each statement or answers the question.
A small gas laser of the type used in classrooms may radiate light at a power level of . If the wavelength of the laser light is , how many photons does it emit per second?
A small gas laser of the type used in classrooms may radiate light at a power level of . If the wavelength of the laser light is , how many photons does it emit per second?

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
42
Choose the one alternative that best completes the statement or answers the question.
What frequency of electromagnetic radiation has photons of energy ? 10-34 )
A)
B)
C)
D)
What frequency of electromagnetic radiation has photons of energy ? 10-34 )
A)
B)
C)
D)

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
43
Choose the one alternative that best completes the statement or answers the question.
Which of the following statements are true for the Bohr model of the atom? (There could be more than one correct choice.)
A) There is no general pattern in the spacing of the shells or their energy differences.
B) As we look at higher and higher electron shells, they get farther and farther apart, but the difference in energy between them gets smaller and smaller.
C) The spacing between all the electron shells is the same.
D) As we look at higher and higher electron shells, they get closer and closer together, but the difference in energy between them gets greater and greater.
E) The energy difference between all the electron shells is the same.
Which of the following statements are true for the Bohr model of the atom? (There could be more than one correct choice.)
A) There is no general pattern in the spacing of the shells or their energy differences.
B) As we look at higher and higher electron shells, they get farther and farther apart, but the difference in energy between them gets smaller and smaller.
C) The spacing between all the electron shells is the same.
D) As we look at higher and higher electron shells, they get closer and closer together, but the difference in energy between them gets greater and greater.
E) The energy difference between all the electron shells is the same.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
44
Choose the one alternative that best completes the statement or answers the question.
If the sunlight from a star peaks at a wavelength of , what temperature does this imply for the surface of that star? The constant in Wien's law is .
A)
B)
C)
D)
E)
If the sunlight from a star peaks at a wavelength of , what temperature does this imply for the surface of that star? The constant in Wien's law is .
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
45
Write the word or phrase that best completes each statement or answers the question.
At what rate are photons emitted by a 50.0-W sodium vapor lamp if it is producing monochromatic light of wavelength
At what rate are photons emitted by a 50.0-W sodium vapor lamp if it is producing monochromatic light of wavelength

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
46
Choose the one alternative that best completes the statement or answers the question.
What is the frequency of the most intense radiation from an object with temperature ? The constant in Wien's law is
A)
B)
C)
D)
What is the frequency of the most intense radiation from an object with temperature ? The constant in Wien's law is
A)
B)
C)
D)

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
47
Choose the one alternative that best completes the statement or answers the question.
An ionized atom having Z protons has had all but one of its electrons removed. If R is the radius of the ground state electron orbit in atomic hydrogen, then what is the radius of the shell of the
Remaining electron in the ionized atom? A)
B)
C)
D)
E)
An ionized atom having Z protons has had all but one of its electrons removed. If R is the radius of the ground state electron orbit in atomic hydrogen, then what is the radius of the shell of the
Remaining electron in the ionized atom? A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
48
Write the word or phrase that best completes each statement or answers the question.
What is the wavelength of a photon having energy )
What is the wavelength of a photon having energy )

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
49
Write the word or phrase that best completes each statement or answers the question.
The human eye can just detect green light of wavelength if it arrives at the retina at the rate of . How many photons arrive each second?
The human eye can just detect green light of wavelength if it arrives at the retina at the rate of . How many photons arrive each second?

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
50
Choose the one alternative that best completes the statement or answers the question.
What is the wavelength of the most intense light emitted by a giant star of surface temperature 5000 K? The constant in Wien's law is 0.00290 m · K.
A) 580 nm
B) 582 nm
C) 578 nm
D) 576 nm
What is the wavelength of the most intense light emitted by a giant star of surface temperature 5000 K? The constant in Wien's law is 0.00290 m · K.
A) 580 nm
B) 582 nm
C) 578 nm
D) 576 nm

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
51
Choose the one alternative that best completes the statement or answers the question.
What is the photon energy of red light having a wavelength of )
A)
B)
C)
D)
What is the photon energy of red light having a wavelength of )
A)
B)
C)
D)

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
52
Choose the one alternative that best completes the statement or answers the question.
The surface temperature of the star is 6000 K. At what wavelength is its light output a maximum? The constant in Wien's law is 0.00290 m · K.
A) 850 nm
B) 483 nm
C) 502 nm
D) 311 nm
E) 907 nm
The surface temperature of the star is 6000 K. At what wavelength is its light output a maximum? The constant in Wien's law is 0.00290 m · K.
A) 850 nm
B) 483 nm
C) 502 nm
D) 311 nm
E) 907 nm

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
53
Choose the one alternative that best completes the statement or answers the question.
What is the surface temperature of a star, if its radiation peak occurs at a frequency of ? , and the constant in Wien's law is
A)
B)
C)
D)
E)
What is the surface temperature of a star, if its radiation peak occurs at a frequency of ? , and the constant in Wien's law is
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
54
Choose the one alternative that best completes the statement or answers the question.
How much energy is carried by a photon of light having frequency ? . s)
A)
B)
C)
D)
How much energy is carried by a photon of light having frequency ? . s)
A)
B)
C)
D)

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
55
Write the word or phrase that best completes each statement or answers the question.
An x-ray tube accelerates electrons through a potential difference of . If an electron in the beam suddenly give up its energy in a collision, what is the shortest wavelength x-ray it could produce?
An x-ray tube accelerates electrons through a potential difference of . If an electron in the beam suddenly give up its energy in a collision, what is the shortest wavelength x-ray it could produce?

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
56
Write the word or phrase that best completes each statement or answers the question.
The cosmic background radiation permeating the universe has the spectrum of a blackbody radiator. What is the peak wavelength of this radiation? The constant in Wien's law is .
The cosmic background radiation permeating the universe has the spectrum of a blackbody radiator. What is the peak wavelength of this radiation? The constant in Wien's law is .

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
57
Write the word or phrase that best completes each statement or answers the question.
What are the wavelength and the corresponding photon energy (in electron-volts) of the primary light emitted by an ideal blackbody at each of the following temperatures? , and the constant in Wein's law is )
(a) ?
(b) ?
(c) ?
What are the wavelength and the corresponding photon energy (in electron-volts) of the primary light emitted by an ideal blackbody at each of the following temperatures? , and the constant in Wein's law is )
(a) ?
(b) ?
(c) ?

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
58
Choose the one alternative that best completes the statement or answers the question.
Each photon in a beam of light has an energy of . What is the wavelength of this light? )
A)
B)
C)
D)
E)
Each photon in a beam of light has an energy of . What is the wavelength of this light? )
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
59
Choose the one alternative that best completes the statement or answers the question.
What is the energy (in eV) of an optical photon of frequency , )
A)
B)
C)
D)
What is the energy (in eV) of an optical photon of frequency , )
A)
B)
C)
D)

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
60
Write the word or phrase that best completes each statement or answers the question.
If the surface of our bodies is at what wavelength does the radiation that we emit
peak if we behave like a blackbody? The constant in Wien's law is 0.0029 m · K.
If the surface of our bodies is at what wavelength does the radiation that we emit
peak if we behave like a blackbody? The constant in Wien's law is 0.0029 m · K.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
61
Write the word or phrase that best completes each statement or answers the question.
A photoelectric surface has a work function of . Calculate the maximum kinetic energy, in , of electrons ejected from this surface by electromagnetic radiation of wavelength
A photoelectric surface has a work function of . Calculate the maximum kinetic energy, in , of electrons ejected from this surface by electromagnetic radiation of wavelength

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
62
Write the word or phrase that best completes each statement or answers the question.
If the Iongest wavelength of light that is able to dislodge electrons from a metal is , what is the work function of that metal, in electron-volts?
If the Iongest wavelength of light that is able to dislodge electrons from a metal is , what is the work function of that metal, in electron-volts?

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
63
Choose the one alternative that best completes the statement or answers the question.
For what wavelength does a laser beam deliver photons in one second?
A)
B)
C)
D)
For what wavelength does a laser beam deliver photons in one second?
A)
B)
C)
D)

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
64
Choose the one alternative that best completes the statement or answers the question.
Light with a frequency of is incident on a metal that has a work function of . What is the maximum kinetic energy that a photoelectron ejected in this process can have?
A)
B)
C)
D)
E)
Light with a frequency of is incident on a metal that has a work function of . What is the maximum kinetic energy that a photoelectron ejected in this process can have?
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
65
Choose the one alternative that best completes the statement or answers the question.
Gamma rays are photons with very high energy. How many visible-light photons with a wavelength of would you need to equal the energy of a gamma-ray photon with energy ?
A)
B)
C)
D)
Gamma rays are photons with very high energy. How many visible-light photons with a wavelength of would you need to equal the energy of a gamma-ray photon with energy ?
A)
B)
C)
D)

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
66
Choose the one alternative that best completes the statement or answers the question.
A helium-neon laser emits light at . If the laser emits photons/second, what is its power output in
A)
B)
C)
D)
A helium-neon laser emits light at . If the laser emits photons/second, what is its power output in
A)
B)
C)
D)

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
67
Choose the one alternative that best completes the statement or answers the question.
A laser pulse of duration has a total energy of . If the wavelength of this radiation is 567 , how many photons are emitted in one pulse?
A)
B)
C)
D)
E)
A laser pulse of duration has a total energy of . If the wavelength of this radiation is 567 , how many photons are emitted in one pulse?
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
68
Write the word or phrase that best completes each statement or answers the question.
A metallic surface is illuminated with light of wavelength . If the work function for this metal is , what is the maximum kinetic energy of the ejected electrons, in electron-volts?
A metallic surface is illuminated with light of wavelength . If the work function for this metal is , what is the maximum kinetic energy of the ejected electrons, in electron-volts?

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
69
Choose the one alternative that best completes the statement or answers the question.
What is the longest wavelength of light that can cause photoelectron emission from a metal that has a work function of
A)
B)
C)
D)
E)
What is the longest wavelength of light that can cause photoelectron emission from a metal that has a work function of
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
70
Choose the one alternative that best completes the statement or answers the question.
Light with a wavelength of is incident on a metal that has a work function of . What is the maximum kinetic energy that a photoelectron ejected in this process can have?
A)
B)
C)
D)
E)
Light with a wavelength of is incident on a metal that has a work function of . What is the maximum kinetic energy that a photoelectron ejected in this process can have?
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
71
Choose the one alternative that best completes the statement or answers the question.
The work function of a particular metal is . What is the photoelectric cutoff (threshold) wavelength for this metal?
A)
B)
C)
D)
The work function of a particular metal is . What is the photoelectric cutoff (threshold) wavelength for this metal?
A)
B)
C)
D)

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
72
Choose the one alternative that best completes the statement or answers the question.
Gamma rays are photons with very high energy. What is the wavelength of a gamma-ray photon with energy ?
A)
B)
C)
D)
Gamma rays are photons with very high energy. What is the wavelength of a gamma-ray photon with energy ?
A)
B)
C)
D)

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
73
Choose the one alternative that best completes the statement or answers the question.
An 84-kW AM radio station broadcasts at . How many photons are emitted each second by the transmitting antenna?
A)
B)
C)
D)
An 84-kW AM radio station broadcasts at . How many photons are emitted each second by the transmitting antenna?
A)
B)
C)
D)

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
74
Choose the one alternative that best completes the statement or answers the question.
A metal has a work function of . Find the maximum kinetic energy of the photoelectrons if light of wavelength shines on the metal. × )
A)
B)
C)
D)
A metal has a work function of . Find the maximum kinetic energy of the photoelectrons if light of wavelength shines on the metal. × )
A)
B)
C)
D)

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
75
Write the word or phrase that best completes each statement or answers the question.
A metal surface has a work function of . What is the longest wavelength of light that will eject electrons from the surface of this metal?
A metal surface has a work function of . What is the longest wavelength of light that will eject electrons from the surface of this metal?

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
76
Choose the one alternative that best completes the statement or answers the question.
If the work function of a metal surface is , what frequency of incident light would give a maximum kinetic energy of to the photoelectrons ejected from this surface?
A)
B)
C)
D)
E)
If the work function of a metal surface is , what frequency of incident light would give a maximum kinetic energy of to the photoelectrons ejected from this surface?
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
77
Choose the one alternative that best completes the statement or answers the question.
The work function of a certain metal is . What is the longest wavelength of light that can cause photoelectron emission from this metal? 10-34 J.s)
A)
B)
C)
D)
E)
The work function of a certain metal is . What is the longest wavelength of light that can cause photoelectron emission from this metal? 10-34 J.s)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
78
Choose the one alternative that best completes the statement or answers the question.
What is the wavelength of a photon? )
A)
B)
C)
D)
What is the wavelength of a photon? )
A)
B)
C)
D)

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
79
Choose the one alternative that best completes the statement or answers the question.
What is the cutoff (threshold) frequency for a metal surface that has a work function of ? (1
A)
B)
C)
D)
E)
What is the cutoff (threshold) frequency for a metal surface that has a work function of ? (1
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
80
Choose the one alternative that best completes the statement or answers the question.
A laser emits a pulse of light that lasts . The light has a wavelength of , and each pulse has an energy of . How many photons are emitted in each pulse?
A)
B)
C)
D)
A laser emits a pulse of light that lasts . The light has a wavelength of , and each pulse has an energy of . How many photons are emitted in each pulse?
A)
B)
C)
D)

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck


