Exam 27: Early Quantum Theory and Models of the Atom
Exam 1: Introduction, Measurement, Estimating29 Questions
Exam 2: Describing Motion: Kinematics in One Dimension527 Questions
Exam 3: Kinematics in Two Dimensions; Vectors183 Questions
Exam 4: Dynamics: Newtons Laws of Motion146 Questions
Exam 5: Circular Motion; Gravitation105 Questions
Exam 6: Work and Energy153 Questions
Exam 7: Linear Momentum139 Questions
Exam 8: Rotational Motion148 Questions
Exam 9: Static Equilibrium; Elasticity and Fracture83 Questions
Exam 10: Fluids98 Questions
Exam 11: Oscillations and Waves114 Questions
Exam 12: Sound21 Questions
Exam 13: Temperature and Kinetic Theory87 Questions
Exam 14: Heat88 Questions
Exam 15: The Laws of Thermodynamics78 Questions
Exam 16: Electric Charge and Electric Field99 Questions
Exam 17: Electric Potential107 Questions
Exam 18: Electric Currents96 Questions
Exam 19: Dc Circuits384 Questions
Exam 20: Magnetism164 Questions
Exam 21: Electromagnetic Induction and Faradays Law60 Questions
Exam 22: Electromagnetic Waves167 Questions
Exam 23: Light: Geometric Optics144 Questions
Exam 24: The Wave Nature of Light58 Questions
Exam 25: Optical Instruments156 Questions
Exam 26: The Special Theory of Relativity126 Questions
Exam 27: Early Quantum Theory and Models of the Atom192 Questions
Exam 28: Quantum Mechanics of Atoms74 Questions
Exam 29: Molecules and Solids26 Questions
Exam 30: Nuclear Physics and Radioactivity153 Questions
Exam 31: Nuclear Energy; Effects and Uses of Radiation36 Questions
Exam 32: Elementary Particles19 Questions
Exam 33: Astrophysics and Cosmology25 Questions
Select questions type
For a certain metal, light of frequency is just barely able to dislodge photoelectrons from the metal. )
(a) What will be the stopping potential if light of frequency is shone on the metal?
(b) What is the work function (in electron-volts) of this metal?
Free
(Short Answer)
4.8/5  (36)
(36)
Correct Answer:
(a) 0.625 V (b) 3.00 eV
Consider the Bohr model for the hydrogen atom in its second excited state.
(a) Determine the binding energy of the electron.
(b) What is the radius of the electron orbit, given that ?
(c) How far is it from the next higher excited orbit?
Free
(Short Answer)
4.8/5  (40)
(40)
Correct Answer:
(a)
(b)
(c)
The work function of a certain metal is . What is the longest wavelength of light that can cause photoelectron emission from this metal? 10>-34 J.s)
Free
(Multiple Choice)
4.8/5  (33)
(33)
Correct Answer:
C
Monochromatic light is incident on a metal surface, and the ejected electrons give rise to a current in the circuit shown in the figure. The maximum kinetic energy of the ejected electrons is determined by applying a reverse ('stopping') potential, sufficient to reduce the current in the ammeter to zero. If the intensity of the incident light is increased, how will the required stopping potential change?
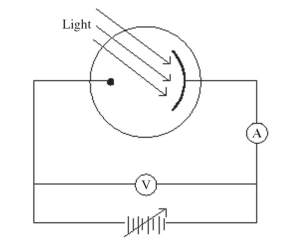
(Multiple Choice)
4.9/5  (36)
(36)
A proton and an electron are both accelerated to the same final kinetic energy. If is the de Broglie wavelength of the proton and is the de Broglie wavelength of the electron, then
(Multiple Choice)
4.8/5  (32)
(32)
What is the shortest wavelength of a photon that can be emitted by a hydrogen atom, for which the initial state is
(Multiple Choice)
4.9/5  (37)
(37)
What is the momentum of a photon of light that has a wavelength of ?
(Multiple Choice)
4.9/5  (33)
(33)
When it is struck by photons, a material having a work function of emits electrons. What is the maximum kinetic energy of the emitted electrons? ( )
(Multiple Choice)
4.8/5  (37)
(37)
Hydrogen atoms can emit four spectral lines with visible colors from red to violet. These four visible lines emitted by hydrogen atoms are produced by electrons
(Multiple Choice)
4.8/5  (35)
(35)
If the de Broglie wavelength of an electron is , what is the speed of this electron? (melectron
(Multiple Choice)
4.9/5  (36)
(36)
The electrons in a beam are moving at . ( )
(a) What is its de Broglie wavelength these electrons?
(b) If the electron beam falls normally on a diffraction grating, what would have to be the spacing between slits in the grating to give a first-order maximum at an angle of with the normal to the grating?
(Short Answer)
4.9/5  (35)
(35)
A photocathode having a work function of is illuminated with monochromatic electromagnetic radiation whose photon energy is . What is the threshold (cutoff) frequency for photoelectron production?
(Multiple Choice)
4.8/5  (36)
(36)
A hydrogen atom with a barely bound electron may have an average radius as large as a bacterium, which is a radius of . What is the nearest principal quantum number of the atom in this state? The radius for ground state hydrogen is .
(Multiple Choice)
4.8/5  (34)
(34)
A crystal diffracts a beam of electrons, like a diffraction grating, as they hit it perpendicular to its surface. The crystal spacing is , and the first maximum scattering occurs at relative to the normal to the surface. ectron
(a) What is the wavelength of the electrons?
(b) What potential difference accelerated the electrons if they started from rest?
(Short Answer)
4.8/5  (36)
(36)
The wavelength of the emitted photon if an electron in the hydrogen atom makes a transition from the state to the ground state is closest to which of the following values? )
(Multiple Choice)
5.0/5  (38)
(38)
Increasing the brightness of a beam of light without changing its color will increase
(Multiple Choice)
4.9/5  (31)
(31)
A highly ionized atom with has only one electron left around it, in its ground state. How much more energy is required to finish the job and have a bare nucleus?
(Multiple Choice)
4.8/5  (33)
(33)
Showing 1 - 20 of 192
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)