Deck 20: Introduction to Carbonyl Chemistry;
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/29
Play
Full screen (f)
Deck 20: Introduction to Carbonyl Chemistry;
1
What is the product of the following reaction?
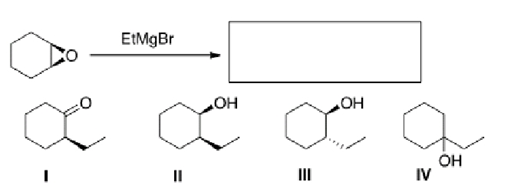
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV
III
2
Which reagent can be used to reduce the alkene in cyclopentenone?
A)NaBH4
B)LiAlH4
C)H2 and Pd-C
D)DIBAL-H
A)NaBH4
B)LiAlH4
C)H2 and Pd-C
D)DIBAL-H
H2 and Pd-C
3
Why would the alcohol in the following compound need to be protected before reaction?
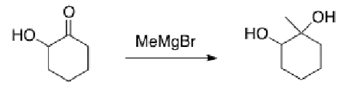
A)If it isn't protected, the product will be a carboxylic acid.
B)The Grignard reagent will react with the alcohol before the ketone.
C)Magnesium is Lewis acidic and will coordinate with the alcohol.
D)There is no need to protect the alcohol.

A)If it isn't protected, the product will be a carboxylic acid.
B)The Grignard reagent will react with the alcohol before the ketone.
C)Magnesium is Lewis acidic and will coordinate with the alcohol.
D)There is no need to protect the alcohol.
The Grignard reagent will react with the alcohol before the ketone.
4
What reagent can be used to cleave a silyl ether protecting group?
A)NaOMe
B)MeMgBr
C)Bu4NF
D)Pd/C
A)NaOMe
B)MeMgBr
C)Bu4NF
D)Pd/C

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
5
What is the product of the following reaction?
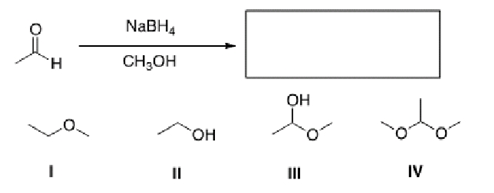
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
6
What will be the product of the following reaction (before any aqueous work-up)?
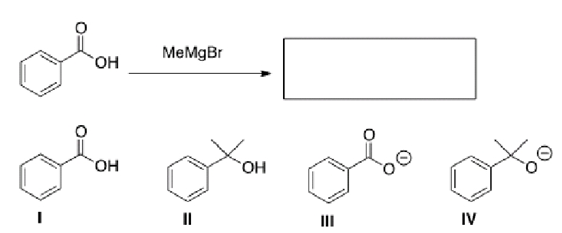
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
7
What is the major organic product in the following sequence of reactions?
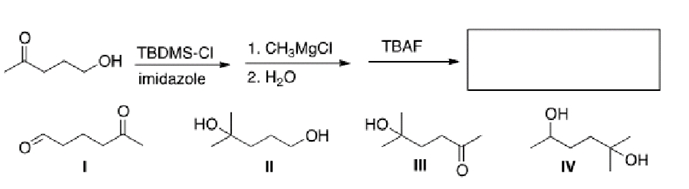
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
8
What is the major organic product of the following reaction?
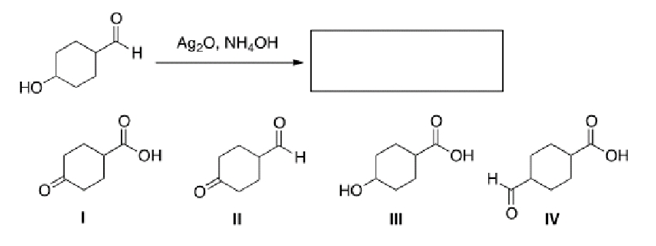
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
9
What is the product of the following reaction?
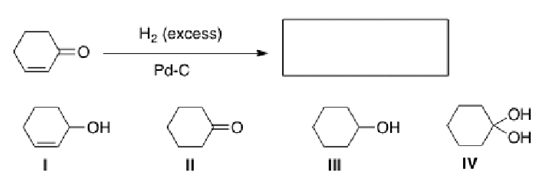
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
10
What is the major organic product of the following reaction?
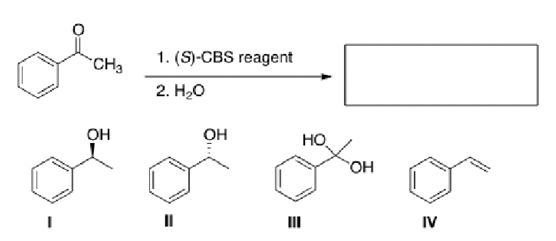
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
11
What is the purpose of a silyl ether?
A)To protect esters from organometallic reagents and other reagents
B)To protect ketones from organometallic reagents and other reagents
C)To protect alcohols from organometallic reagents and other reagents
D)To prevent the formation of carboxylic acids
A)To protect esters from organometallic reagents and other reagents
B)To protect ketones from organometallic reagents and other reagents
C)To protect alcohols from organometallic reagents and other reagents
D)To prevent the formation of carboxylic acids

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
12
What is the major organic product of the following reaction?

A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
13
Why are ketones less reactive than aldehydes?
A)Ketones are more sterically hindered.
B)Ketones are less electron deficient due to donation from the two alkyl groups.
C)The statement is false; ketones are more reactive than aldehydes.
D)Both (a) Ketones are more sterically hindered and (b) Ketones are less electron deficient due to donation from the two alkyl groups.
A)Ketones are more sterically hindered.
B)Ketones are less electron deficient due to donation from the two alkyl groups.
C)The statement is false; ketones are more reactive than aldehydes.
D)Both (a) Ketones are more sterically hindered and (b) Ketones are less electron deficient due to donation from the two alkyl groups.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
14
What is the product of the following reaction?
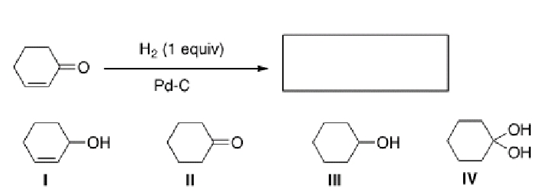
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
15
What is the product of the following reaction?
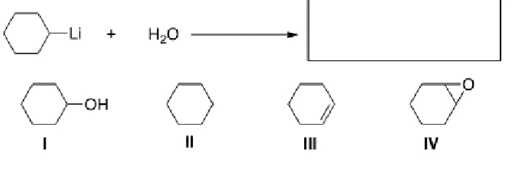
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
16
Which reagent can be used to reduce an acid chloride to an aldehyde?
A)NaBH4
B)LiAlH(OtBu)3
C)LiAlH4
D)FeCl3
A)NaBH4
B)LiAlH(OtBu)3
C)LiAlH4
D)FeCl3

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
17
What is the name of the general reaction type that aldehydes and ketones undergo?
A)Electrophilic addition
B)Nucleophilic addition
C)Nucleophilic substitution
D)Electrophilic substitution
A)Electrophilic addition
B)Nucleophilic addition
C)Nucleophilic substitution
D)Electrophilic substitution

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
18
What reagent would be used to reduce an amide to an amine?
A)NaBH4
B)LiAlH(OtBu)3
C)LiAlH4
D)FeCl3
A)NaBH4
B)LiAlH(OtBu)3
C)LiAlH4
D)FeCl3

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
19
Which reagent can be used to reduce the carbonyl in methyl vinyl ketone?
A)NaBH4/CH3OH
B)H2 and Pd-C
C)FeCl3
D)NaH
A)NaBH4/CH3OH
B)H2 and Pd-C
C)FeCl3
D)NaH

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
20
What is the product of the following reaction?
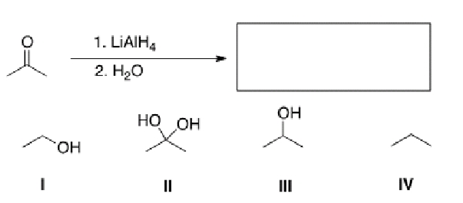
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
21
If the starting material has no stereogenic centers, when carbonyl compounds are reduced with a reagent such as LiAlH4 or NaBH4 and a new stereogenic center is formed, what will the composition of the product mixture be?
A)Forms a racemic mixture of the two possible enantiomers
B)Forms more of one enantiomer than another because of steric reactions around the carbonyl
C)Forms more of one enantiomer than another depending on the temperature of the reaction
D)Forms different products depending on the solvent used
A)Forms a racemic mixture of the two possible enantiomers
B)Forms more of one enantiomer than another because of steric reactions around the carbonyl
C)Forms more of one enantiomer than another depending on the temperature of the reaction
D)Forms different products depending on the solvent used

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
22
What is the major organic product of the following reaction?
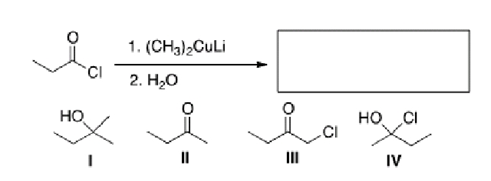
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
23
What is the starting material in the reaction below?
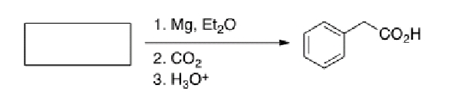
A)Bromobenzene
B)Benzyl bromide
C)Benzoic acid
D)Lithium benzoate

A)Bromobenzene
B)Benzyl bromide
C)Benzoic acid
D)Lithium benzoate

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
24
What is the missing reagent in the reaction below?
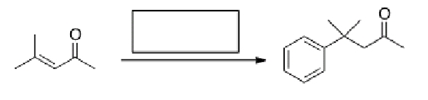
A)PhMgBr then H2O
B)Benzene, AlCl3
C)Ph2CuLi then H2O
D)PhLi then H2O

A)PhMgBr then H2O
B)Benzene, AlCl3
C)Ph2CuLi then H2O
D)PhLi then H2O

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
25
What is the missing reagent in the reaction below?
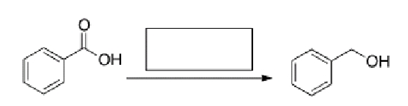
A)NaBH4/CH3OH
B)H2, Pd-C
C)LiAlH4 then H2O
D)MeMgBr then H2O

A)NaBH4/CH3OH
B)H2, Pd-C
C)LiAlH4 then H2O
D)MeMgBr then H2O

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
26
What is the missing reagent in the reaction below?
![<strong>What is the missing reagent in the reaction below? </strong> A)[1] LiAlH<sub>4</sub>, [2] H<sub>2</sub>O B)[1] CH<sub>2</sub>=CHLi, [2] H<sub>2</sub>O C)[1] (CH<sub>2</sub>=CH)<sub>2</sub>CuLi, [2] H<sub>2</sub>O D)[1] DIBAL-H, [2] H<sub>2</sub>O](https://storage.examlex.com/TB5872/11ec712e_a962_5228_8ed2_1ba7fbf7a687_TB5872_00.jpg)
A)[1] LiAlH4, [2] H2O
B)[1] CH2=CHLi, [2] H2O
C)[1] (CH2=CH)2CuLi, [2] H2O
D)[1] DIBAL-H, [2] H2O
![<strong>What is the missing reagent in the reaction below? </strong> A)[1] LiAlH<sub>4</sub>, [2] H<sub>2</sub>O B)[1] CH<sub>2</sub>=CHLi, [2] H<sub>2</sub>O C)[1] (CH<sub>2</sub>=CH)<sub>2</sub>CuLi, [2] H<sub>2</sub>O D)[1] DIBAL-H, [2] H<sub>2</sub>O](https://storage.examlex.com/TB5872/11ec712e_a962_5228_8ed2_1ba7fbf7a687_TB5872_00.jpg)
A)[1] LiAlH4, [2] H2O
B)[1] CH2=CHLi, [2] H2O
C)[1] (CH2=CH)2CuLi, [2] H2O
D)[1] DIBAL-H, [2] H2O

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
27
Both LiAlH4 and NaBH4 are reducing agents.Which statement about these reagents is true?
A)Both reagents contain polar metal-hydrogen bonds.The polarity of the B-H bond is greater than the polarity of the Al-H bond, so LiAlH4 is the stronger reducing agent.
B)Both reagents contain polar metal-hydrogen bonds.The polarity of the B-H bond is greater than the polarity of the Al-H bond, so LiAlH4 is the weaker reducing agent.
C)Both reagents contain polar metal-hydrogen bonds.The polarity of the B-H bond is less than the polarity of the Al-H bond, so LiAlH4 is the stronger reducing agent.
D)Both reagents contain polar metal-hydrogen bonds.The polarity of the B-H bond is less than the polarity of the Al-H bond, so LiAlH4 is the weaker reducing agent.
A)Both reagents contain polar metal-hydrogen bonds.The polarity of the B-H bond is greater than the polarity of the Al-H bond, so LiAlH4 is the stronger reducing agent.
B)Both reagents contain polar metal-hydrogen bonds.The polarity of the B-H bond is greater than the polarity of the Al-H bond, so LiAlH4 is the weaker reducing agent.
C)Both reagents contain polar metal-hydrogen bonds.The polarity of the B-H bond is less than the polarity of the Al-H bond, so LiAlH4 is the stronger reducing agent.
D)Both reagents contain polar metal-hydrogen bonds.The polarity of the B-H bond is less than the polarity of the Al-H bond, so LiAlH4 is the weaker reducing agent.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
28
What is the missing reagent in the reaction below?
![<strong>What is the missing reagent in the reaction below? </strong> A)[1] LiAlH<sub>4</sub>, [2] H<sub>2</sub>O B)[1] CH<sub>3</sub>MgBr (excess), [2] H<sub>2</sub>O C)[1] (CH<sub>3</sub>)<sub>2</sub>CuLi (excess), [2] H<sub>2</sub>O D)[1] DIBAL-H, [2] H<sub>2</sub>O](https://storage.examlex.com/TB5872/11ec712e_8b45_1f77_8ed2_dbb60504d577_TB5872_00.jpg)
A)[1] LiAlH4, [2] H2O
B)[1] CH3MgBr (excess), [2] H2O
C)[1] (CH3)2CuLi (excess), [2] H2O
D)[1] DIBAL-H, [2] H2O
![<strong>What is the missing reagent in the reaction below? </strong> A)[1] LiAlH<sub>4</sub>, [2] H<sub>2</sub>O B)[1] CH<sub>3</sub>MgBr (excess), [2] H<sub>2</sub>O C)[1] (CH<sub>3</sub>)<sub>2</sub>CuLi (excess), [2] H<sub>2</sub>O D)[1] DIBAL-H, [2] H<sub>2</sub>O](https://storage.examlex.com/TB5872/11ec712e_8b45_1f77_8ed2_dbb60504d577_TB5872_00.jpg)
A)[1] LiAlH4, [2] H2O
B)[1] CH3MgBr (excess), [2] H2O
C)[1] (CH3)2CuLi (excess), [2] H2O
D)[1] DIBAL-H, [2] H2O

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
29
A carbonyl group, C=O, and an alkene, C=C, double bonds are both sp2 hybridized.However, the chemistry of These two functional groups is very different.This can be explained by which of the following statements?
A)The bond angle of the carbonyl is larger than the bond angle of the alkene.
B)The electronegative oxygen of the C=O group makes this bond polar.
C)The bond of the C=C is longer that the bond of the C=O.
D)There is more steric crowding in the carbonyl than in the alkene.
A)The bond angle of the carbonyl is larger than the bond angle of the alkene.
B)The electronegative oxygen of the C=O group makes this bond polar.
C)The bond of the C=C is longer that the bond of the C=O.
D)There is more steric crowding in the carbonyl than in the alkene.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck


