Deck 18: Derivatives of Carboxylic Acids: Acyl Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/68
Play
Full screen (f)
Deck 18: Derivatives of Carboxylic Acids: Acyl Compounds
1
Which is the correct name for the following compound?
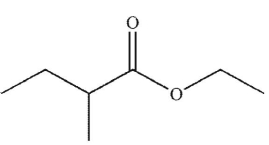
A) methyl 2-methylbutanoate
B) ethyl 2-methylbutanoate
C) ethyl butanoate
D) ethylmethylbutanoate
E) sec-butyl ethanoate

A) methyl 2-methylbutanoate
B) ethyl 2-methylbutanoate
C) ethyl butanoate
D) ethylmethylbutanoate
E) sec-butyl ethanoate
ethyl 2-methylbutanoate
2
Which of these structures is a 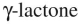 ?
?
A)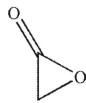
B)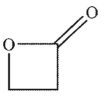
C)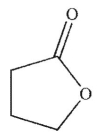
D)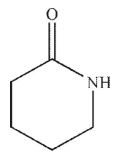
E)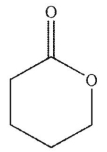
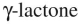 ?
?A)

B)

C)

D)

E)


3
Which of the following compounds will have the highest 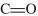 stretching frequency (that is, the highest wave number)?
stretching frequency (that is, the highest wave number)?
A)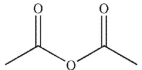
B)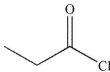
C)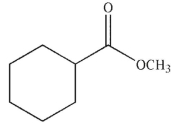
D)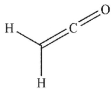
E)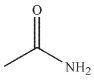
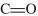 stretching frequency (that is, the highest wave number)?
stretching frequency (that is, the highest wave number)?A)

B)

C)

D)

E)


4
Which of these compounds would have the most downfield carbonyl signal in its 13C NMR spectrum?
A)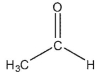
B)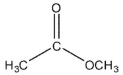
C)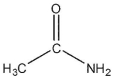
D)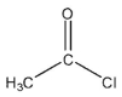
E)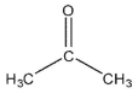
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
5
What is the proper IUPAC name for this compound?
![<strong>What is the proper IUPAC name for this compound? </strong> A) (1S,4S)-bicyclo[2.2.1]heptan-2-amide B) (1S,4R)-3-azabicyclo[2.2.1]heptan-2-one C) (1S,4R)-bicylo[2.2.1]heptan-2-amide D) (1S,2S)-bicyclo[2.2.1]-3-azaheptanone E) (1S,4S)-3-azabicyclo[2.2.1]heptan-2-one](https://storage.examlex.com/TB34225555/11ec819b_dbb9_70b0_96fc_2f7e9e213496_TB34225555_11.jpg)
A) (1S,4S)-bicyclo[2.2.1]heptan-2-amide
B) (1S,4R)-3-azabicyclo[2.2.1]heptan-2-one
C) (1S,4R)-bicylo[2.2.1]heptan-2-amide
D) (1S,2S)-bicyclo[2.2.1]-3-azaheptanone
E) (1S,4S)-3-azabicyclo[2.2.1]heptan-2-one
![<strong>What is the proper IUPAC name for this compound? </strong> A) (1S,4S)-bicyclo[2.2.1]heptan-2-amide B) (1S,4R)-3-azabicyclo[2.2.1]heptan-2-one C) (1S,4R)-bicylo[2.2.1]heptan-2-amide D) (1S,2S)-bicyclo[2.2.1]-3-azaheptanone E) (1S,4S)-3-azabicyclo[2.2.1]heptan-2-one](https://storage.examlex.com/TB34225555/11ec819b_dbb9_70b0_96fc_2f7e9e213496_TB34225555_11.jpg)
A) (1S,4S)-bicyclo[2.2.1]heptan-2-amide
B) (1S,4R)-3-azabicyclo[2.2.1]heptan-2-one
C) (1S,4R)-bicylo[2.2.1]heptan-2-amide
D) (1S,2S)-bicyclo[2.2.1]-3-azaheptanone
E) (1S,4S)-3-azabicyclo[2.2.1]heptan-2-one

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
6
Rank the following acyl compounds in order of increasing  bond length.
bond length.
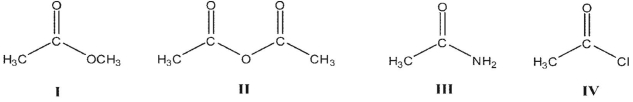
A)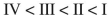
B)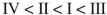
C)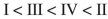
D)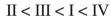
E)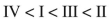
 bond length.
bond length.
A)
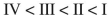
B)
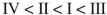
C)
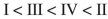
D)
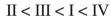
E)
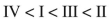

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
7
Which of these carbonyl compounds has the least basic carbonyl oxygen atom?
A)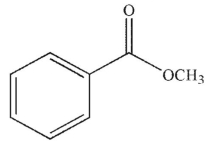
B)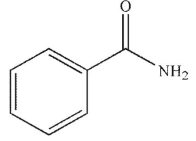
C)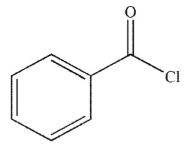
D)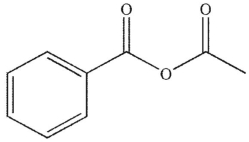
E)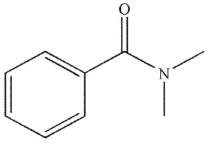
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
8
What is the product of the following synthetic sequence?
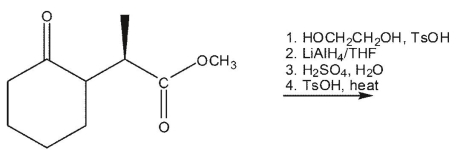
A)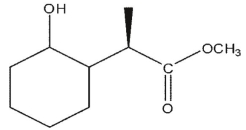
B)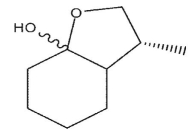
C)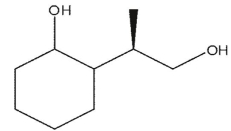
D)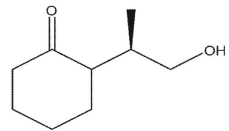
E)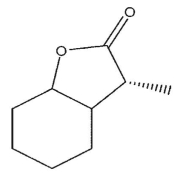

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
9
Place the following compounds in order of increasing resonance stabilization.
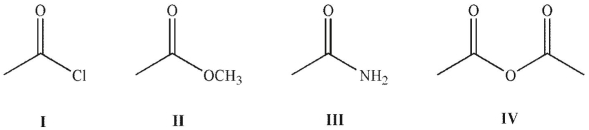
A)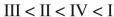
B)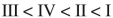
C)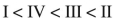
D)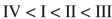
E)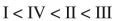

A)
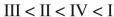
B)
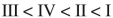
C)
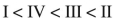
D)
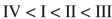
E)
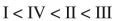

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
10
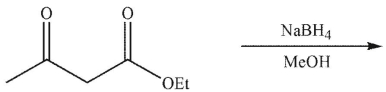
What is the product of the reaction conditions shown?
A)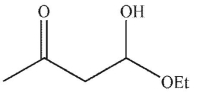
B)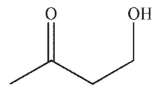
C)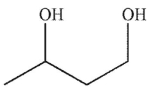
D)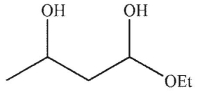
E)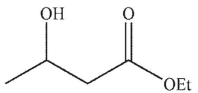

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following structures is (S)-3-methyl-2-oxacyclopent-4-en-1-one?
A)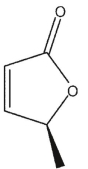
B)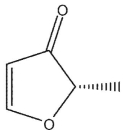
C)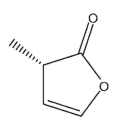
D)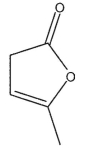
E)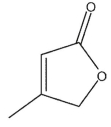
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following reagents would you use to accomplish this transformation?

A) NaBH4
B) LiAlH4
C) DIBAL-H
D) H2
E) H3O+

A) NaBH4
B) LiAlH4
C) DIBAL-H
D) H2
E) H3O+

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
13
Which is the correct name for the following compound?
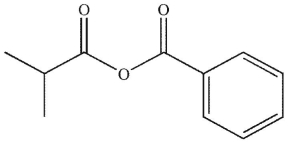
A) 2-methylpropanoic benzoic anhydride
B) benzoic methylpropanoate
C) benzoic 2-methylpropanoic anhydride
D) benzoic propanoic anhydride
E) benzoic isopropanoic anhydride

A) 2-methylpropanoic benzoic anhydride
B) benzoic methylpropanoate
C) benzoic 2-methylpropanoic anhydride
D) benzoic propanoic anhydride
E) benzoic isopropanoic anhydride

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
14
List the reagents in sequence used for the following synthesis.
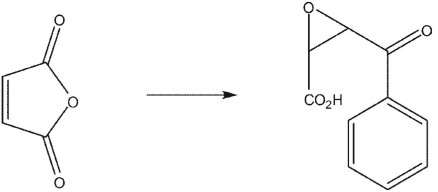
A)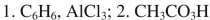
B)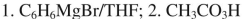
C)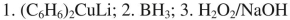
D)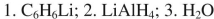
E)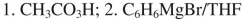

A)
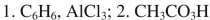
B)
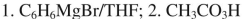
C)
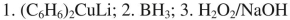
D)
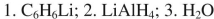
E)
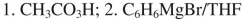

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
15
Which reagent would you use to accomplish the following transformation?

A) PLiAlH4
B) LiBH4
C) NaBH4
D) DIBAL-H
E) Both a and b

A) PLiAlH4
B) LiBH4
C) NaBH4
D) DIBAL-H
E) Both a and b

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following structures is N -methylpropanamide?
A)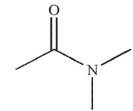
B)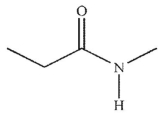
C)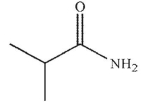
D)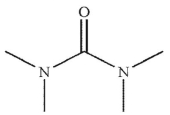
E)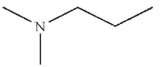
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
17
Predict the product of the following reaction.
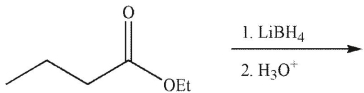
A)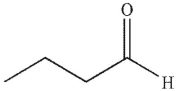
B)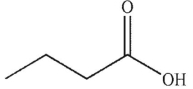
C)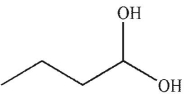
D)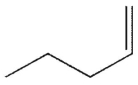
E)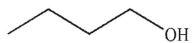

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following compounds cannot be synthesized directly from ethanoyl chloride?
A)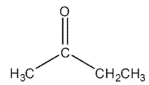
B)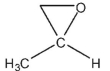
C)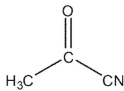
D)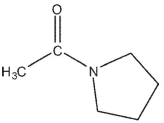
E)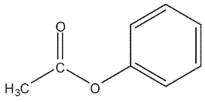
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following reagents would you use to accomplish this transformation?
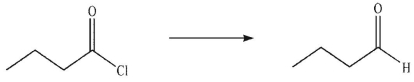
A) LiAlH4
B) Me2CuLi
C)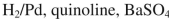
D) CH3MgBr
E) H2O

A) LiAlH4
B) Me2CuLi
C)
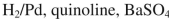
D) CH3MgBr
E) H2O

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following compounds is the major organic product of the reaction conditions shown?
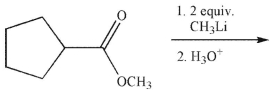
A)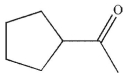
B)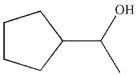
C)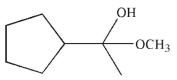
D)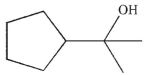
E)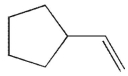

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following is the product of the reaction conditions shown? 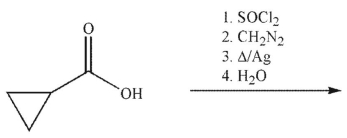
A)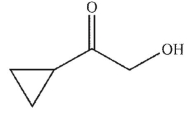
B)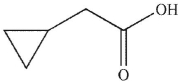
C)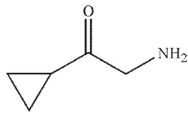
D)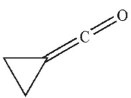
E)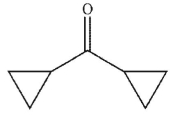

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following is the product of the reaction conditions shown?
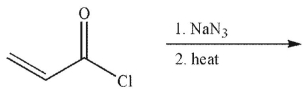
A)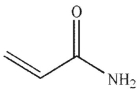
B)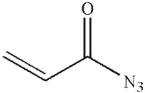
C)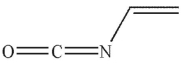
D)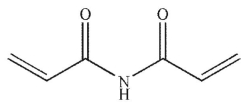
E)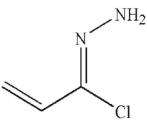
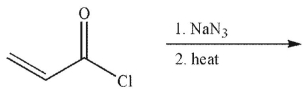
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
23
Provide the correct IUPAC name for the following compound. 


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following is the final product of the reaction conditions shown?
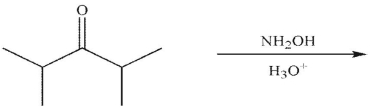
A)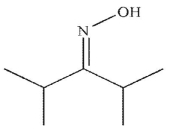
B)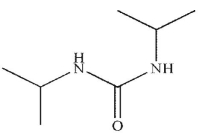
C)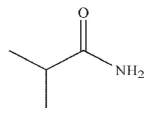
D)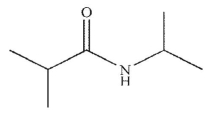
E)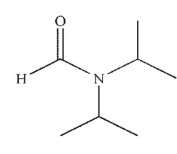

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
25
Draw the following compound: R-N-ethyl-N-methyl-3-hydroxybutanamide.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following is the correct product of the reaction conditions shown?
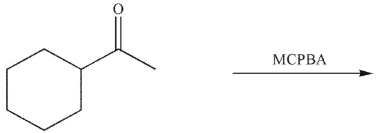
A)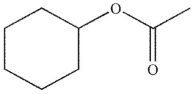
B)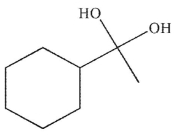
C)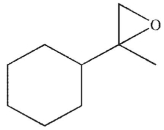
D)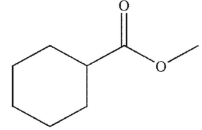
E)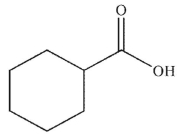

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
27
What reagent would you use to accomplish the following transformation?
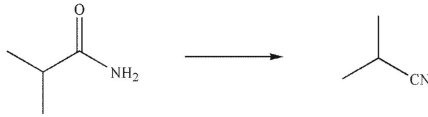
A) H2/Pd
B) LiAlH4
C) P2O5
D) NaCN , then H3O+
E) None of these reagents could be used for this transformation.

A) H2/Pd
B) LiAlH4
C) P2O5
D) NaCN , then H3O+
E) None of these reagents could be used for this transformation.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
28
What is the product of the following sequence of reactions?
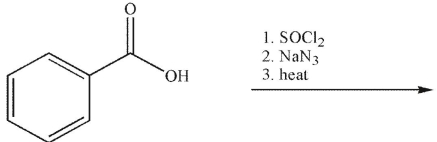
A)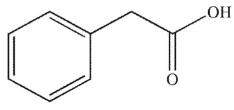
B)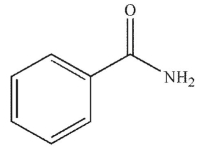
C)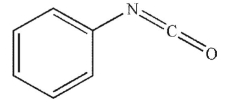
D)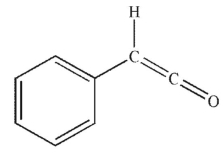
E)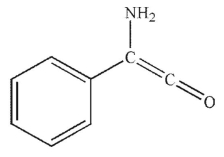

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
29
Which has more resonance stabilization, an ester or an acid chloride? Explain your answer.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
30
Why is acidic amide hydrolysis irreversible?
A)The amine leaving group is protonated, forming an ammonium ion.
B)The amide carbonyl is protonated.
C)The amine leaving group is deprotonated, forming an amide ion.
D)The carboxylic acid formed in the reaction is deprotonated.
E)The amine formed in the reaction is deprotonated.
A)The amine leaving group is protonated, forming an ammonium ion.
B)The amide carbonyl is protonated.
C)The amine leaving group is deprotonated, forming an amide ion.
D)The carboxylic acid formed in the reaction is deprotonated.
E)The amine formed in the reaction is deprotonated.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
31
For compound A, in acid, it is the hydroxyl oxygen that is protonated to a greater extent than the
carbonyl oxygen.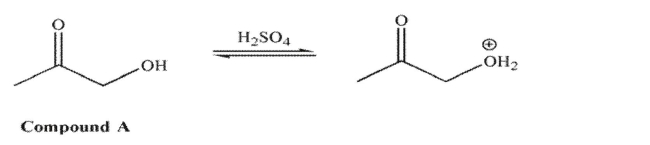 However, for compound B in acid, it is the carbonyl oxygen that is protonated to a greater extent
However, for compound B in acid, it is the carbonyl oxygen that is protonated to a greater extent
than the hydroxyl oxygen.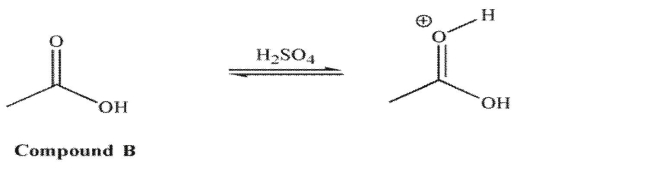 Explain the difference in these results.
Explain the difference in these results.
carbonyl oxygen.
 However, for compound B in acid, it is the carbonyl oxygen that is protonated to a greater extent
However, for compound B in acid, it is the carbonyl oxygen that is protonated to a greater extentthan the hydroxyl oxygen.
 Explain the difference in these results.
Explain the difference in these results.
Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
32
Draw the structure of methyl 3,3-dimethylbutanoate.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following compounds will not be transformed to a carboxylic acid on treatment with aqueous acid?
A)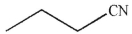
B)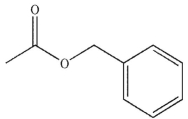
C)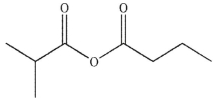
D)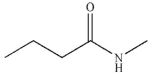
E) All these compounds can be transformed to carboxylic acids on treatment with aqueous acid.
A)

B)

C)

D)

E) All these compounds can be transformed to carboxylic acids on treatment with aqueous acid.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
34
What conditions could be used to accomplish the following transformation? 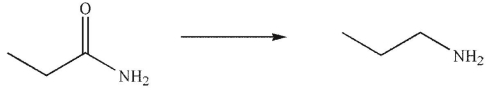
A)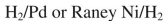
B)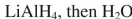
C) Br2 , then aqueous NaOH and heat
D) NaBH4 in methanol solvent
E) both b and c

A)
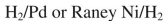
B)
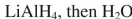
C) Br2 , then aqueous NaOH and heat
D) NaBH4 in methanol solvent
E) both b and c

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
35
Provide an acceptable name for the structure shown here. 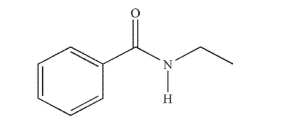


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
36
What is the final product of the reaction conditions shown?
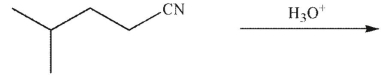
A)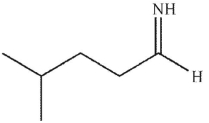
B)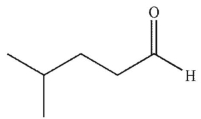
C)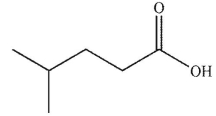
D)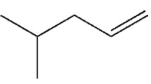
E)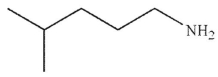
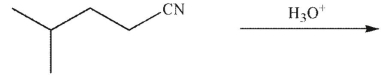
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following is the correct product of the reaction conditions shown?
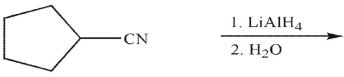
A)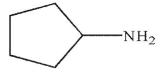
B)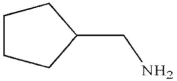
C)
D)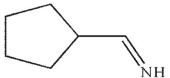
E)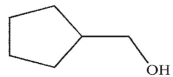
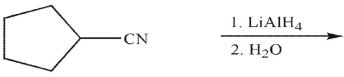
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following statements about amide hydrolysis is false?
A)Both acidic and basic pathways are addition-elimination reactions.
B)Under acidic conditions, the leaving group is ammonia or an amine.
C)Under basic conditions, the leaving group is an amide ion.
D)In both acidic and basic pathways, the amide's carbonyl oxygen is protonated.
E)Both acidic and basic amide hydrolysis pathways are irreversible.
A)Both acidic and basic pathways are addition-elimination reactions.
B)Under acidic conditions, the leaving group is ammonia or an amine.
C)Under basic conditions, the leaving group is an amide ion.
D)In both acidic and basic pathways, the amide's carbonyl oxygen is protonated.
E)Both acidic and basic amide hydrolysis pathways are irreversible.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following is not a product of the hydrolysis of the compound shown?
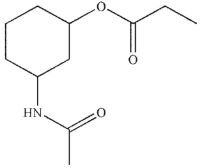
A)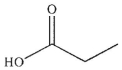
B)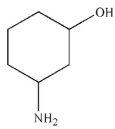
C)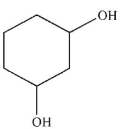
D)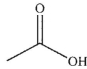
E)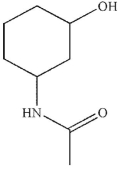

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following is an isolable intermediate in the reaction shown?
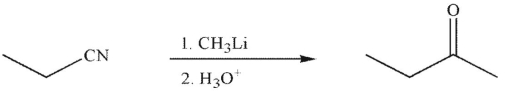
A)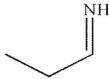
B)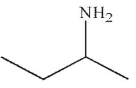
C)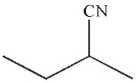
D)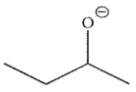
E)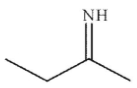
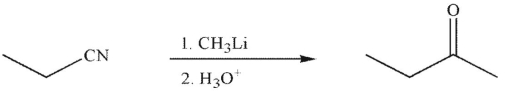
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
41
Match the compound shown to the correct NMR spectrum. 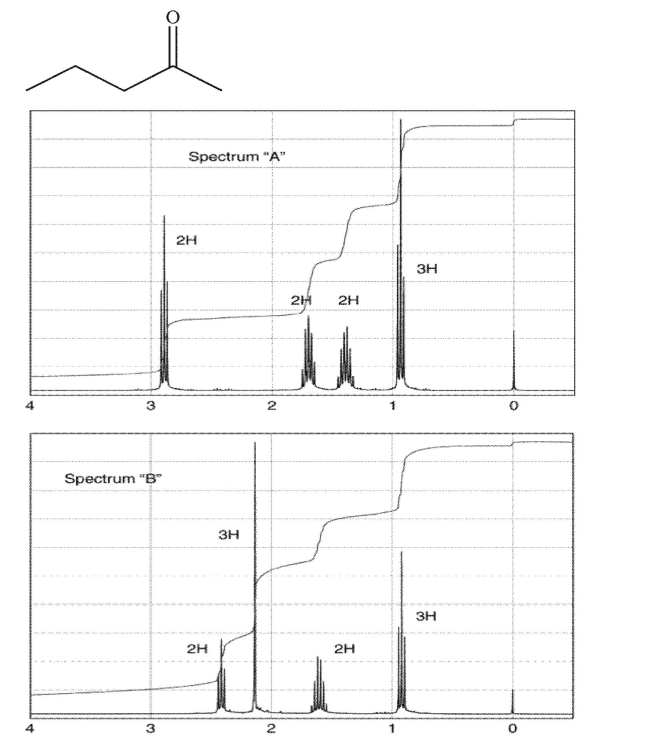


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
42
Predict the product of the following reaction conditions. 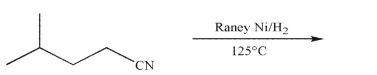


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
43
List the reagents required to convert benzoyl chloride to each product shown. 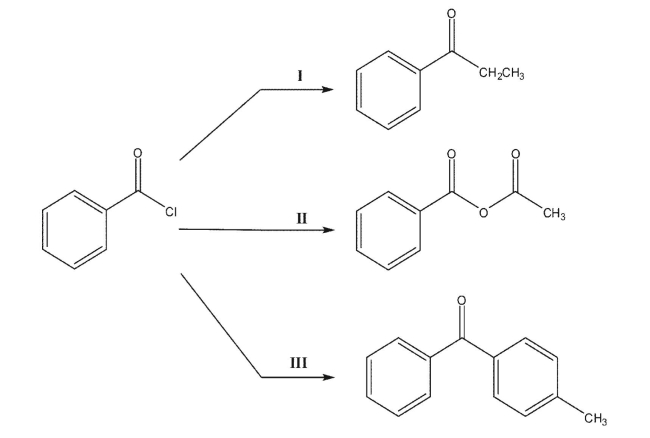


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
44
Draw a mechanism for the following transformation.Include all necessary lone pairs, curved
arrows, and nonzero formal charges.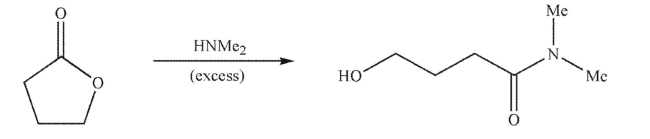
arrows, and nonzero formal charges.


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
45
For the following reaction: 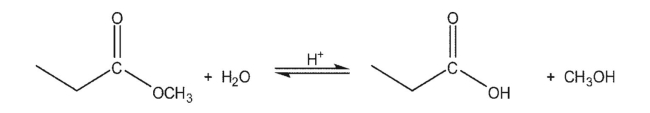 How would each of the following affect the position of the equilibrium?
How would each of the following affect the position of the equilibrium?
A)Running the reaction in methanol as a solvent
B)Running the reaction in a still at a temperature of 85-90 °C
C)Running the reaction in the presence of anhydrous sodium sulfate at room temperature
 How would each of the following affect the position of the equilibrium?
How would each of the following affect the position of the equilibrium? A)Running the reaction in methanol as a solvent
B)Running the reaction in a still at a temperature of 85-90 °C
C)Running the reaction in the presence of anhydrous sodium sulfate at room temperature

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
46
Draw the structure of the compound with molecular formula C4H4O3 that has the following spectra.
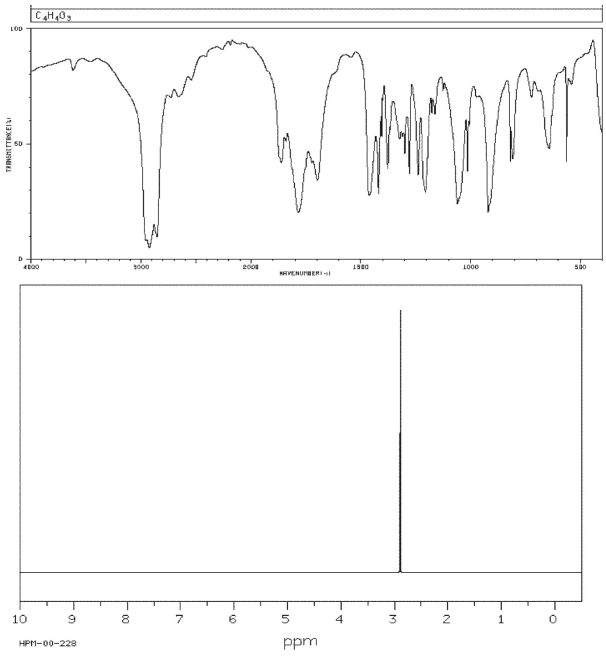


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
47
Predict the major organic product of the reaction conditions shown here and draw a mechanism to
rationalize its formation.Include all necessary lone pairs, curved arrows, and nonzero formal
charges.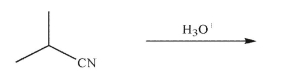
rationalize its formation.Include all necessary lone pairs, curved arrows, and nonzero formal
charges.
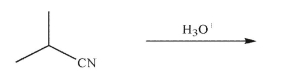

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
48
Using  as your only carbon source, provide syntheses of each of the following molecules.
as your only carbon source, provide syntheses of each of the following molecules.
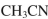 as your only carbon source, provide syntheses of each of the following molecules.
as your only carbon source, provide syntheses of each of the following molecules.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
49
Draw a mechanism for the transformation shown here.Include all necessary lone pairs of
electrons, curved arrows, and nonzero formal charges.
electrons, curved arrows, and nonzero formal charges.


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
50
Predict the product of the following reaction. 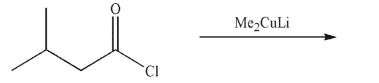
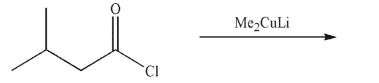

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the compounds shown here matches this NMR spectrum? 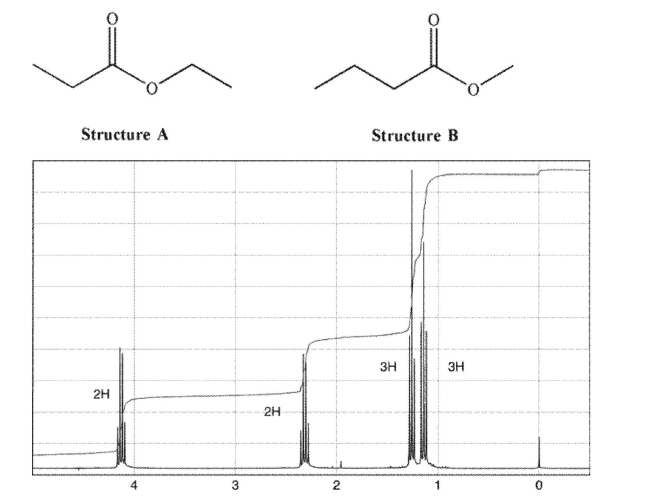


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
52
Predict the major organic product of the following reaction conditions and draw a mechanism to
rationalize its formation.Include all necessary lone pairs of electrons, curved arrows, and nonzero
formal charges.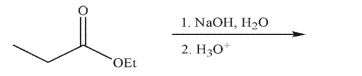
rationalize its formation.Include all necessary lone pairs of electrons, curved arrows, and nonzero
formal charges.
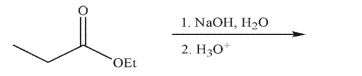

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
53
Predict the product of the reaction shown. 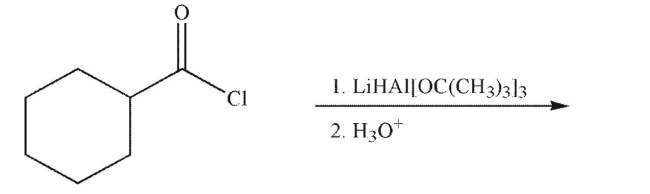


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
54
Predict the product of the following reaction conditions. 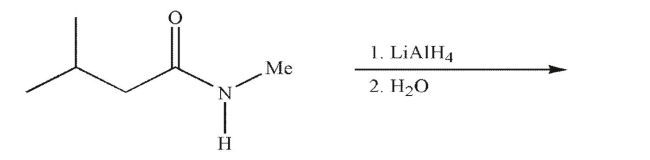


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
55
Show the product of the following reaction sequence. 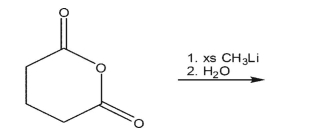


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
56
Draw a mechanism for the transformation shown here.Include all necessary lone pairs of
electrons, curved arrows, and nonzero formal charges.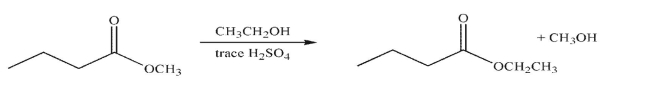
electrons, curved arrows, and nonzero formal charges.


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
57
Devise a multistep synthesis of the target molecule from the starting material shown.Show the
reagents needed for each step and the product of each step.Do not show any mechanisms.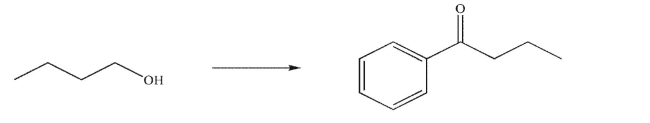
reagents needed for each step and the product of each step.Do not show any mechanisms.


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
58
Provide the structures of two carboxylic acid derivatives from which the compound shown here
could be synthesized, along with the reagents you would use to convert each to the alcohol.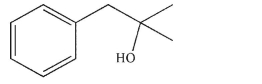
could be synthesized, along with the reagents you would use to convert each to the alcohol.


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
59
Devise a multistep synthesis of the target material from the starting material shown.Show the
reagents needed for each step and the product of each step.Do not draw any mechanisms.
reagents needed for each step and the product of each step.Do not draw any mechanisms.


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
60
Predict the product of the following reaction. 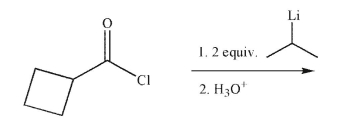


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
61
Show the intermediates and final product in the following synthesis. 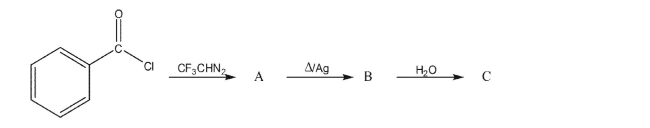


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
62
Draw the mechanism and final product for the Baeyer-Villiger reaction of methyl cyclopentyl
ketone.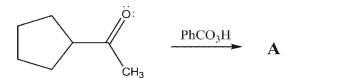
ketone.


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
63
The lactone shown can be synthesized in one step from two different precursors.Draw them both
and provide the reagents necessary to transform each of them into the lactone.
and provide the reagents necessary to transform each of them into the lactone.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
64
Predict the product of the following reaction conditions and draw a mechanism to rationalize its
formation.Include all necessary lone pairs of electrons, curved arrows, and nonzero formal
charges.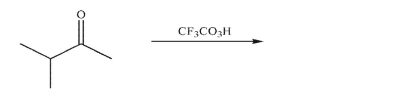
formation.Include all necessary lone pairs of electrons, curved arrows, and nonzero formal
charges.


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
65
Devise a multistep synthesis of the target molecule from the starting material shown.Show the
reagents needed for each step and the product of each step.Do not show any mechanisms.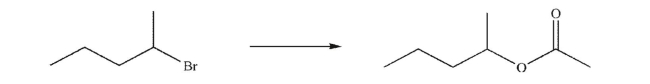
reagents needed for each step and the product of each step.Do not show any mechanisms.


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
66
Predict the product of the reaction conditions shown. 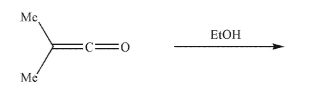


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
67
Predict the product of the following reaction conditions and draw a mechanism to rationalize its
formation.Include all necessary lone pairs of electrons, curved arrows, and nonzero formal
charges.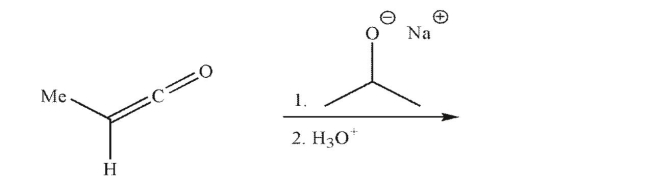
formation.Include all necessary lone pairs of electrons, curved arrows, and nonzero formal
charges.


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
68
Draw a mechanism for the transformation shown here.Include all necessary lone pairs of
electrons, curved arrows, and nonzero formal charges.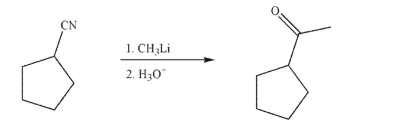
electrons, curved arrows, and nonzero formal charges.


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck


