Exam 18: Derivatives of Carboxylic Acids: Acyl Compounds
Exam 1: Atoms and Molecules; Orbitals and Bonding64 Questions
Exam 2: Alkanes65 Questions
Exam 3: Alkenes and Alkynes70 Questions
Exam 4: Stereochemistry68 Questions
Exam 5: Rings60 Questions
Exam 6: Substituted Alkanes: Alkyl Halides, Alcohols, Amines, Ethers,thiols, and Thioethers68 Questions
Exam 7: Substitution Reactions: the Sn2 and Sn1 Reactions55 Questions
Exam 8: Elimination Reactions: the E1 and E2 Reactions45 Questions
Exam 9: Analytical Chemistry: Spectroscopy65 Questions
Exam 10: Electrophilic Additions to Alkenes68 Questions
Exam 11: More Additions to Bonds65 Questions
Exam 12: Radical Reactions65 Questions
Exam 13: Dienes and the Allyl System: 2p Orbitals in Conjugation68 Questions
Exam 14: Aromaticity66 Questions
Exam 15: Substitution Reactions of Aromatic Compounds68 Questions
Exam 16: Carbonyl Chemistry 1: Addition Reactions73 Questions
Exam 17: Carboxylic Acids66 Questions
Exam 18: Derivatives of Carboxylic Acids: Acyl Compounds68 Questions
Exam 19: Carbonyl Chemistry 2: Reactions at the Α Position71 Questions
Exam 20: Special Topic: Carbohydrates40 Questions
Exam 21: Special Topic: Bio-Organic Chemistry40 Questions
Exam 22: Special Topic: Amino Acids and Polyamino Acids Peptides and Proteins39 Questions
Exam 23: Special Topic: Reactions Controlled by Orbital Symmetry46 Questions
Exam 24: Special Topic: Intramolecular Reactions and Neighboring Group Participation40 Questions
Select questions type
Which of the following is the correct product of the reaction conditions shown?
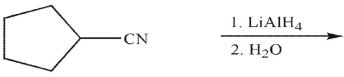
Free
(Multiple Choice)
4.9/5  (27)
(27)
Correct Answer:
B
Provide the structures of two carboxylic acid derivatives from which the compound shown here
could be synthesized, along with the reagents you would use to convert each to the alcohol. 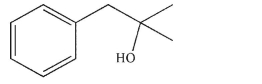
Free
(Essay)
4.8/5  (51)
(51)
Correct Answer:
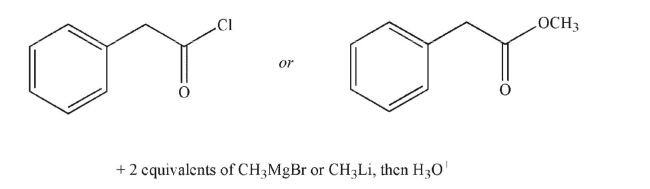
Which of the following is the product of the reaction conditions shown? 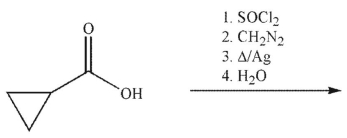
Free
(Multiple Choice)
4.8/5  (40)
(40)
Correct Answer:
B
Which of the following is an isolable intermediate in the reaction shown?
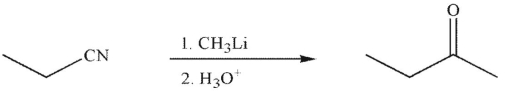
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following reagents would you use to accomplish this transformation?

(Multiple Choice)
4.9/5  (37)
(37)
Devise a multistep synthesis of the target material from the starting material shown.Show the
reagents needed for each step and the product of each step.Do not draw any mechanisms. 
(Essay)
4.8/5  (37)
(37)
Which has more resonance stabilization, an ester or an acid chloride? Explain your answer.
(Essay)
4.8/5  (39)
(39)
Devise a multistep synthesis of the target molecule from the starting material shown.Show the
reagents needed for each step and the product of each step.Do not show any mechanisms. 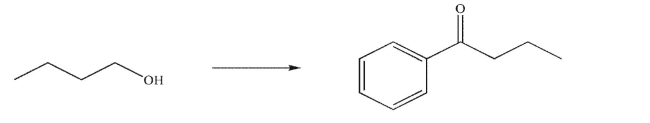
(Essay)
4.9/5  (46)
(46)
Predict the product of the following reaction conditions and draw a mechanism to rationalize its
formation.Include all necessary lone pairs of electrons, curved arrows, and nonzero formal
charges. 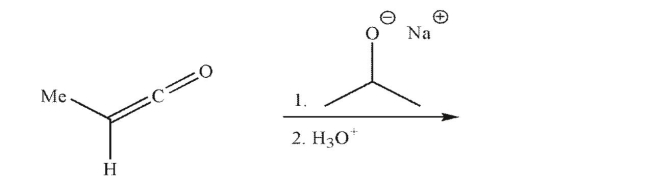
(Essay)
4.7/5  (45)
(45)
Predict the major organic product of the following reaction conditions and draw a mechanism to
rationalize its formation.Include all necessary lone pairs of electrons, curved arrows, and nonzero
formal charges. 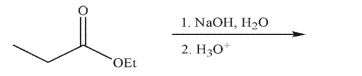
(Essay)
4.8/5  (33)
(33)
Which of the following compounds is the major organic product of the reaction conditions shown?
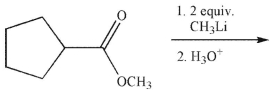
(Multiple Choice)
4.9/5  (46)
(46)
For compound A, in acid, it is the hydroxyl oxygen that is protonated to a greater extent than the
carbonyl oxygen. 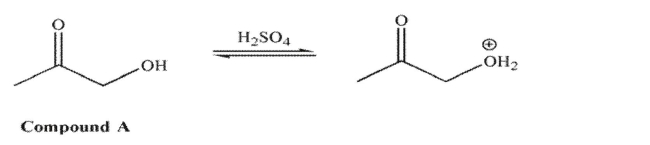 However, for compound B in acid, it is the carbonyl oxygen that is protonated to a greater extent
than the hydroxyl oxygen.
However, for compound B in acid, it is the carbonyl oxygen that is protonated to a greater extent
than the hydroxyl oxygen. 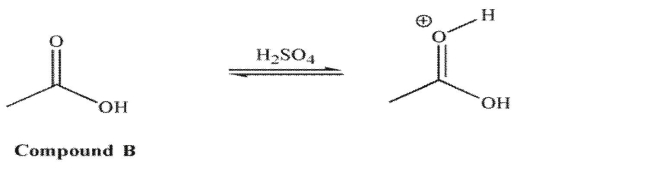 Explain the difference in these results.
Explain the difference in these results.
(Essay)
4.7/5  (43)
(43)
Which reagent would you use to accomplish the following transformation?

(Multiple Choice)
4.8/5  (28)
(28)
What conditions could be used to accomplish the following transformation? 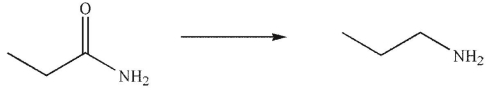
(Multiple Choice)
4.9/5  (34)
(34)
Place the following compounds in order of increasing resonance stabilization.
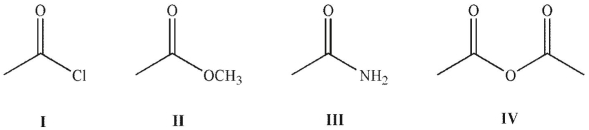
(Multiple Choice)
4.8/5  (47)
(47)
List the reagents required to convert benzoyl chloride to each product shown. 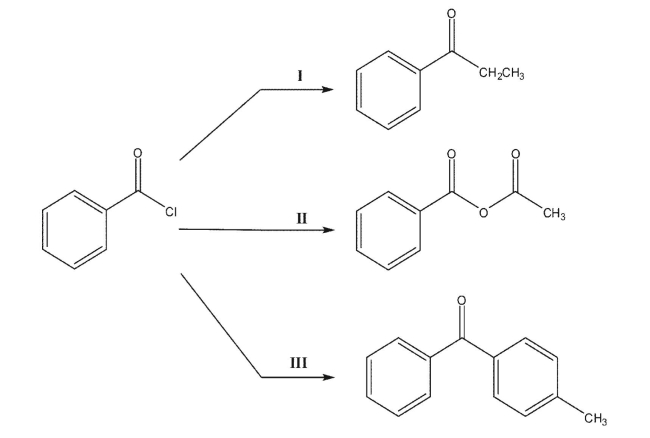
(Essay)
4.8/5  (43)
(43)
Showing 1 - 20 of 68
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)