Deck 5: Gases, Liquids, and Solids
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/104
Play
Full screen (f)
Deck 5: Gases, Liquids, and Solids
1
A gas occupies a volume of 6 L at 3 atm pressure. Calculate the volume of the gas when the pressure increases to 9 atm at the same temperature.
A) 0.5 L
B) 1 L
C) 4 L
D) 2 L
A) 0.5 L
B) 1 L
C) 4 L
D) 2 L
D
2
Which of the following laws relates the pressure and the temperature of a gas when volume is kept constant?
A) Boyle's law
B) Charles's law
C) Dalton's law
D) Gay-Lussac's law
A) Boyle's law
B) Charles's law
C) Dalton's law
D) Gay-Lussac's law
D
3
Which of the following leads to an increase in the kinetic energy of a molecule?
A) an increase in surface area
B) an increase in temperature
C) an increase in density
D) an increase in intermolecular attractive forces
A) an increase in surface area
B) an increase in temperature
C) an increase in density
D) an increase in intermolecular attractive forces
B
4
Which state of matter has the weakest attractive forces between its molecules?
A) A solid has the weakest attractive forces between its molecules.
B) A liquid has the weakest attractive forces between its molecules.
C) A gas has the weakest attractive forces between its molecules.
D) The attractive forces between the molecules are the same for all the states of matter.
A) A solid has the weakest attractive forces between its molecules.
B) A liquid has the weakest attractive forces between its molecules.
C) A gas has the weakest attractive forces between its molecules.
D) The attractive forces between the molecules are the same for all the states of matter.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
5
Which physical state of matter is represented by the following model? 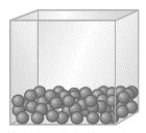
A) solid
B) liquid
C) gas

A) solid
B) liquid
C) gas

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following directly determine the physical state of a substance at a given temperature?
A) covalent bonds
B) intermolecular forces
C) intramolecular forces
D) nuclear forces
A) covalent bonds
B) intermolecular forces
C) intramolecular forces
D) nuclear forces

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is the SI unit of pressure?
A) bars
B) atmospheres
C) torr
D) pascal
A) bars
B) atmospheres
C) torr
D) pascal

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following instruments is used to measure atmospheric pressure?
A) an altimeter
B) a barometer
C) a manometer
D) a thermometer
A) an altimeter
B) a barometer
C) a manometer
D) a thermometer

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following laws relates the volume and the pressure of a gas when temperature is kept constant?
A) Avogadro's law
B) Boyle's law
C) Charles's law
D) Dalton's law
A) Avogadro's law
B) Boyle's law
C) Charles's law
D) Dalton's law

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
10
When the state of a substance changes from A to B as represented by the given models, which of the following statements is true? 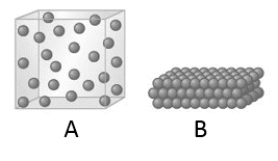
A) The velocity of the molecules increases.
B) The strength of the intermolecular forces increases.
C) The kinetic energy of the molecules decreases.
D) The strength of the intermolecular force increases and the kinetic energy of the molecules decreases.

A) The velocity of the molecules increases.
B) The strength of the intermolecular forces increases.
C) The kinetic energy of the molecules decreases.
D) The strength of the intermolecular force increases and the kinetic energy of the molecules decreases.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is a mathematical representation of Boyle's law?
A) P1V2 = P2V1
B) P1V1 = P2V2
C) P1/T2 = P2/T1
D) P1/T1 = P2/T2
A) P1V2 = P2V1
B) P1V1 = P2V2
C) P1/T2 = P2/T1
D) P1/T1 = P2/T2

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
12
At constant pressure, the temperature of a 3 L liquid is 56°C. Calculate the temperature if the volume of the liquid changes to 5 L.
A) 93 K
B) 548 K
C) 349°C
D) 440°C
A) 93 K
B) 548 K
C) 349°C
D) 440°C

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
13
The temperature of a substance is decreased to a low value. Which of the following models would represent this substance at the lowered temperature? 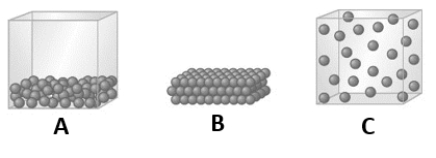
A) A
B) B
C) C

A) A
B) B
C) C

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
14
What is the average pressure of the atmosphere at sea level?
A) 760 mm Hg
B) 35.92 in. Hg
C) 780 torr
D) 760 bars
A) 760 mm Hg
B) 35.92 in. Hg
C) 780 torr
D) 760 bars

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following laws relates the volume and the temperature of a gas when pressure is kept constant?
A) Avogadro's law
B) Boyle's law
C) Charles's law
D) Dalton's law
A) Avogadro's law
B) Boyle's law
C) Charles's law
D) Dalton's law

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following units can be used to measure air pressure?
A) atmospheres
B) millimeters of Hg
C) torr
D) all of these
A) atmospheres
B) millimeters of Hg
C) torr
D) all of these

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following gas laws is related to the process of respiration?
A) Avogadro's law
B) Boyle's law
C) Charles's law
D) Dalton's law
A) Avogadro's law
B) Boyle's law
C) Charles's law
D) Dalton's law

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is not a unit of pressure?
A) millimeters of Hg
B) pascals
C) torr
D) hertz
A) millimeters of Hg
B) pascals
C) torr
D) hertz

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following increases as temperature increases?
A) kinetic energy
B) intermolecular attractive forces
C) molecular weight
D) none of these
A) kinetic energy
B) intermolecular attractive forces
C) molecular weight
D) none of these

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following instruments is used to measure the pressure of a gas sample in a container?
A) an altimeter
B) a barometer
C) a manometer
D) a thermometer
A) an altimeter
B) a barometer
C) a manometer
D) a thermometer

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
21
What will happen when the temperature of a gas is increased from 20°C to 40°C at constant pressure?
A) The volume of the gas will double.
B) The volume of the gas will decrease by half.
C) The volume of the gas will decrease slightly.
D) The volume of the gas will increase slightly.
A) The volume of the gas will double.
B) The volume of the gas will decrease by half.
C) The volume of the gas will decrease slightly.
D) The volume of the gas will increase slightly.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
22
Suppose a balloon is filled so that its volume is 2.00 L when the pressure is 750 torr and the temperature is 24°C. What is the volume of the balloon when it rises to an elevation where the pressure is 375 torr and the temperature is 12°C?
A) 0.261 L
B) 0.500 L
C) 2.00 L
D) 3.84 L
A) 0.261 L
B) 0.500 L
C) 2.00 L
D) 3.84 L

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following is a mathematical representation of the combined gas law?
A) P1V2/T1 = P2V1/T2
B) P1V2/T2 = P2V1/T1
C) P1V1/T2 = P2V2/T1
D) P1V1/T1 = P2V2/T2
A) P1V2/T1 = P2V1/T2
B) P1V2/T2 = P2V1/T1
C) P1V1/T2 = P2V2/T1
D) P1V1/T1 = P2V2/T2

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following is a mathematical representation of Gay-Lussac's law?
A) P1/V2 = P2/V1
B) P1/V1 = P2/V2
C) P1/T2 = P2/T1
D) P1/T1 = P2/T2
A) P1/V2 = P2/V1
B) P1/V1 = P2/V2
C) P1/T2 = P2/T1
D) P1/T1 = P2/T2

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following is a mathematical representation of Boyle's law?
A) P1/V2 = P2/V1
B) P1/V1 = P2/V2
C) P1/T2 = P2/T1
D) P1/T1 = P2/T2
A) P1/V2 = P2/V1
B) P1/V1 = P2/V2
C) P1/T2 = P2/T1
D) P1/T1 = P2/T2

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following is true if the mercury level is the same on both sides of the J tube in a manometer?
A) The gas pressure is higher than the atmospheric pressure.
B) The gas pressure is lower than the atmospheric pressure.
C) The gas pressure is equal to the atmospheric pressure.
D) The volume of the gas is directly proportional to atmospheric pressure.
A) The gas pressure is higher than the atmospheric pressure.
B) The gas pressure is lower than the atmospheric pressure.
C) The gas pressure is equal to the atmospheric pressure.
D) The volume of the gas is directly proportional to atmospheric pressure.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following is a mathematical representation of Gay-Lussac's law?
A) P1V2 = P2V1
B) P1V1 = P2V2
C) P1T2 = P2T1
D) P1T1 = P2T2
A) P1V2 = P2V1
B) P1V1 = P2V2
C) P1T2 = P2T1
D) P1T1 = P2T2

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
28
At constant pressure, the temperature of a 4.00 L sample of gas is increased from 300 K to 400 K. Calculate the new volume of the gas sample.
A) 0.188 L
B) 0.300 L
C) 3.33 L
D) 5.33 L
A) 0.188 L
B) 0.300 L
C) 3.33 L
D) 5.33 L

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
29
The temperature of a 6.5 L sample of a gas at constant pressure is 50°C. Calculate the temperature when the volume of the gas sample increases to 6.8 L.
A) 358°C
B) 338°C
C) 358 K
D) 338 K
A) 358°C
B) 338°C
C) 358 K
D) 338 K

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
30
At constant pressure, the temperature of a 4.00 L sample of gas is increased from 25°C to 50°C. Calculate the new volume of the gas sample.
A) 2.00 L
B) 3.69 L
C) 4.34 L
D) 8.00 L
A) 2.00 L
B) 3.69 L
C) 4.34 L
D) 8.00 L

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
31
At constant pressure, the temperature of a 4.0 L sample of gas is decreased from 50°C to 25°C. Calculate the new volume of the gas sample.
A) 2.00 L
B) 3.69 L
C) 4.34 L
D) 8.00 L
A) 2.00 L
B) 3.69 L
C) 4.34 L
D) 8.00 L

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
32
At constant temperature, the pressure on a 10.0 L sample of gas is changed from 1.00 atm to 1140 torr. Calculate the new volume of the gas sample.
A) 0.00877 L
B) 6.67 L
C) 15.0 L
D) 114 L
A) 0.00877 L
B) 6.67 L
C) 15.0 L
D) 114 L

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
33
Suppose a balloon is filled so that its volume is 2.00 L when the pressure is 1.10 atm and the temperature is 300 K. What is the volume of the balloon when it rises to an elevation where the pressure is 418 mm Hg and the temperature is 200 K?
A) 3.30 L
B) 2.67 L
C) 0.375 L
D) 0.303 L
A) 3.30 L
B) 2.67 L
C) 0.375 L
D) 0.303 L

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
34
At constant temperature, the pressure on a 10.0 L sample of gas is changed from 1140 torr to 1.00 atm. Calculate the new volume of the gas sample.
A) 0.00877 L
B) 6.67 L
C) 15.0 L
D) 114 L
A) 0.00877 L
B) 6.67 L
C) 15.0 L
D) 114 L

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
35
At constant pressure, the temperature of a 4.00 L sample of gas is decreased from 400 K to 300 K. Calculate the new volume of the gas sample.
A) 0.188 L
B) 0.333 L
C) 3.00 L
D) 5.33 L
A) 0.188 L
B) 0.333 L
C) 3.00 L
D) 5.33 L

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following is a mathematical representation of Charles's law?
A) P1V2 = P2V1
B) P1V1 = P2V2
C) V1T2 = V2T1
D) V1T1 = V2T2
A) P1V2 = P2V1
B) P1V1 = P2V2
C) V1T2 = V2T1
D) V1T1 = V2T2

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
37
At constant temperature, the pressure on a 6.0 L sample of a gas is reduced from 2.0 atm to 1.0 atm. Calculate the new volume of the gas sample.
A) 0.083 L
B) 0.33 L
C) 3.0 L
D) 12 L
A) 0.083 L
B) 0.33 L
C) 3.0 L
D) 12 L

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following is a mathematical representation of Charles's law?
A) P1/V2 = P2/V1
B) P1/V1 = P2/V2
C) V1/T2 = V2/T1
D) V1/T1 = V2/T2
A) P1/V2 = P2/V1
B) P1/V1 = P2/V2
C) V1/T2 = V2/T1
D) V1/T1 = V2/T2

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
39
At constant temperature, the pressure on a 6.0 L sample of gas is increased from 1.0 atm to 2.0 atm. Calculate the new volume of the gas sample.
A) 0.33 L
B) 0.67 L
C) 1.5 L
D) 3.0 L
A) 0.33 L
B) 0.67 L
C) 1.5 L
D) 3.0 L

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following is a mathematical representation of the combined gas law?
A) P1V2T1 = P2V1T2
B) P1V2T2 = P2V1T1
C) P1V1T2 = P2V2T1
D) P1V1T1 = P2V2T2
A) P1V2T1 = P2V1T2
B) P1V2T2 = P2V1T1
C) P1V1T2 = P2V2T1
D) P1V1T1 = P2V2T2

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
41
What will happen when the temperature of a gas is decreased from 60 K to 30 K at constant pressure?
A) The volume of the gas will double.
B) The volume of the gas will decrease by half.
C) The volume of the gas will decrease slightly.
D) The volume of the gas will increase slightly.
A) The volume of the gas will double.
B) The volume of the gas will decrease by half.
C) The volume of the gas will decrease slightly.
D) The volume of the gas will increase slightly.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
42
A student has three samples of gases that have equal volume. The three gases are O2, CO2, and NH3; all the gases are at the same temperature and pressure. Which of the following sample contains the largest number of atoms?
A) O2 contains the largest number of atoms.
B) CO2 contains the largest number of atoms.
C) NH3 contains the largest number of atoms.
D) All three gas samples contain an equal number of atoms.
A) O2 contains the largest number of atoms.
B) CO2 contains the largest number of atoms.
C) NH3 contains the largest number of atoms.
D) All three gas samples contain an equal number of atoms.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following laws relates the volume and the number of molecules of a gas when temperature and pressure are kept constant?
A) Avogadro's law
B) Boyle's law
C) Charles's law
D) Dalton's law
A) Avogadro's law
B) Boyle's law
C) Charles's law
D) Dalton's law

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
44
What will happen when the temperature of a gas is decreased from 60°C to 30°C at constant pressure?
A) The volume of the gas will double.
B) The volume of the gas will decrease by half.
C) The volume of the gas will decrease slightly.
D) The volume of the gas will increase slightly.
A) The volume of the gas will double.
B) The volume of the gas will decrease by half.
C) The volume of the gas will decrease slightly.
D) The volume of the gas will increase slightly.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following are measures of standard temperature and pressure?
A) 0°C and 1 atm
B) 32°F and 1 atm
C) 273 K and 1 atm
D) all of these
A) 0°C and 1 atm
B) 32°F and 1 atm
C) 273 K and 1 atm
D) all of these

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
46
A certain quantity of neon gas is under 1.05 atm pressure at 303 K in a 10.0 L vessel. How many moles of neon are present in the neon gas?
A) 0.222 mol
B) 0.402 mol
C) 0.422 mol
D) 2.37 mol
A) 0.222 mol
B) 0.402 mol
C) 0.422 mol
D) 2.37 mol

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following is a mathematical representation of the ideal gas law?
A) PVRT = n
B) PV/RT = n
C) VRT/P = n
D) n = RT/PV
A) PVRT = n
B) PV/RT = n
C) VRT/P = n
D) n = RT/PV

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
48
In which of the following laws is volume maintained as a constant?
A) Charles's law
B) Avogadro's law
C) Gay-Lussac's law
D) Boyle's law
A) Charles's law
B) Avogadro's law
C) Gay-Lussac's law
D) Boyle's law

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following is a mathematical representation of the ideal gas law?
A) PV = n/RT
B) PV = nRT
C) V = nP/RT
D) PV = RT/n
A) PV = n/RT
B) PV = nRT
C) V = nP/RT
D) PV = RT/n

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
50
If an 8-g sample of molecular oxygen at 1.00 atm pressure occupies 3.00 L, calculate the temperature of the sample.
A) 146°C
B) 146 K
C) 73°C
D) 73 K
A) 146°C
B) 146 K
C) 73°C
D) 73 K

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
51
If 2 mol of NO gas occupies 10.0 L at 295 K, what is the pressure of the gas in atmospheres?
A) 0.206 atm
B) 2.42 atm
C) 4.84 atm
D) 9.33 atm
A) 0.206 atm
B) 2.42 atm
C) 4.84 atm
D) 9.33 atm

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
52
What volume does one mole of oxygen gas occupy at a temperature of 273 K and at a pressure of 1.00 atm?
A) 30.4 L
B) 30 L
C) 22.4 L
D) 20 L
A) 30.4 L
B) 30 L
C) 22.4 L
D) 20 L

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
53
If a 7-g sample of molecular nitrogen occupies 2.50 L at 10°C, calculate the pressure of the sample.
A) 1.28 atm
B) 2.32 atm
C) 2.56 atm
D) 4.65 atm
A) 1.28 atm
B) 2.32 atm
C) 2.56 atm
D) 4.65 atm

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
54
What will happen when the temperature of a gas is increased from 20 K to 40 K at constant pressure?
A) The volume of the gas will double.
B) The volume of the gas will decrease by half.
C) The volume of the gas will decrease slightly.
D) The volume of the gas will increase slightly.
A) The volume of the gas will double.
B) The volume of the gas will decrease by half.
C) The volume of the gas will decrease slightly.
D) The volume of the gas will increase slightly.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
55
A sample of carbon dioxide occupies 22.4 L at STP. Which of the following statements is true of the sample?
A) The sample contains 6.02 × 1023 atoms of carbon.
B) The sample contains 6.02 × 1023 atoms of oxygen.
C) The sample contains 6.02 × 1023 molecules of carbon dioxide.
D) None of these are correct.
A) The sample contains 6.02 × 1023 atoms of carbon.
B) The sample contains 6.02 × 1023 atoms of oxygen.
C) The sample contains 6.02 × 1023 molecules of carbon dioxide.
D) None of these are correct.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
56
A student has three samples of gases that have equal volume. The three gases are O2, CO2, and NH3; all the gases are at the same temperature and pressure. Which of the following samples contains the largest number of molecules?
A) O2 contains the largest number of molecules.
B) CO2 contains the largest number of molecules.
C) NH3 contains the largest number of molecules.
D) All three gas samples contain an equal number of molecules.
A) O2 contains the largest number of molecules.
B) CO2 contains the largest number of molecules.
C) NH3 contains the largest number of molecules.
D) All three gas samples contain an equal number of molecules.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following are measures of standard temperature and pressure?
A) 0°C and 1 atm
B) 0°F and 1 atm
C) 0 K and 1 atm
D) none of these
A) 0°C and 1 atm
B) 0°F and 1 atm
C) 0 K and 1 atm
D) none of these

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
58
Calculate the volume occupied by an 8-g sample of molecular oxygen at STP.
A) 5.60 L
B) 11.2 L
C) 22.4 L
D) 44.8 L
A) 5.60 L
B) 11.2 L
C) 22.4 L
D) 44.8 L

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
59
A gas expands from an initial volume of 20.5 L at 0.92 atm and 23°C to a final volume of 34.6 L. During the expansion, the gas cools to 12°C. Calculate the final pressure of the gas.
A) 0.28 atm
B) 0.52 atm
C) 0.57 atm
D) 1.9 atm
A) 0.28 atm
B) 0.52 atm
C) 0.57 atm
D) 1.9 atm

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following is the correct unit for the ideal gas law constant, R?
A) L/mol
B) L·atm−1·mol
C) L·atm·mol−1·K−1
D) L·atm·mol·K
A) L/mol
B) L·atm−1·mol
C) L·atm·mol−1·K−1
D) L·atm·mol·K

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following increases when the number of collisions per unit time increases for a gas in a container?
A) volume
B) density
C) pressure
D) attractive force between the molecules
A) volume
B) density
C) pressure
D) attractive force between the molecules

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following laws applies only to mixtures of gases?
A) Avogadro's law
B) Boyle's law
C) Charles's law
D) Dalton's law
A) Avogadro's law
B) Boyle's law
C) Charles's law
D) Dalton's law

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
63
Dry air is 78.08% nitrogen, 20.95% oxygen and 0.93% argon with the 0.04% consisting of other gases. Calculate the partial pressure of nitrogen in dry air when the atmospheric pressure is 760 torr.
A) 78.08 torr
B) 166.6 torr
C) 593.4 torr
D) 760.0 torr
A) 78.08 torr
B) 166.6 torr
C) 593.4 torr
D) 760.0 torr

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following is not true of the kinetic molecular theory?
A) The average kinetic energy of gas particles is proportional to the temperature in kelvins.
B) Gas molecules are assumed to have no volume.
C) Gas pressure is caused by collisions between gas molecules and the container walls.
D) The pressure of a gas sample reduces as the number of collisions per unit time increases.
A) The average kinetic energy of gas particles is proportional to the temperature in kelvins.
B) Gas molecules are assumed to have no volume.
C) Gas pressure is caused by collisions between gas molecules and the container walls.
D) The pressure of a gas sample reduces as the number of collisions per unit time increases.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following is not true of the kinetic molecular theory?
A) Gas molecules are assumed to have no volume.
B) Gas pressure is caused by collisions between gas molecules and the container walls.
C) When gas molecules collide, they stick together.
D) None, all of these are true.
A) Gas molecules are assumed to have no volume.
B) Gas pressure is caused by collisions between gas molecules and the container walls.
C) When gas molecules collide, they stick together.
D) None, all of these are true.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
66
An unknown amount of He gas occupies 30.5 L at 2.00 atm pressure and 300 K. Calculate the weight of the gas in the container.
A) 2.48 g
B) 4.95 g
C) 9.91 g
D) 19.8 g
A) 2.48 g
B) 4.95 g
C) 9.91 g
D) 19.8 g

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
67
What is condensation?
A) It is the change of a substance from the liquid state to the gaseous state.
B) It is the change of a substance from the solid state to the liquid state.
C) It is the change of a substance from the liquid state to the solid state.
D) It is the change of a substance from the gaseous state to the liquid state.
A) It is the change of a substance from the liquid state to the gaseous state.
B) It is the change of a substance from the solid state to the liquid state.
C) It is the change of a substance from the liquid state to the solid state.
D) It is the change of a substance from the gaseous state to the liquid state.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following is not an example of an intermolecular attractive force?
A) Covalent bonding
B) Dipole-dipole interactions
C) London dispersion forces
D) Hydrogen bonding
A) Covalent bonding
B) Dipole-dipole interactions
C) London dispersion forces
D) Hydrogen bonding

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following is usually the weakest intermolecular interaction in small molecules?
A) Covalent bonding
B) Dipole-dipole interactions
C) London dispersion forces
D) Hydrogen bonding
A) Covalent bonding
B) Dipole-dipole interactions
C) London dispersion forces
D) Hydrogen bonding

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following might correspond to the O2 pressure in a hyperbaric chamber?
A) 160 torr
B) 760 torr
C) 1600 torr
D) none of these
A) 160 torr
B) 760 torr
C) 1600 torr
D) none of these

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
71
A vessel under 2.015 atm pressure containing nitrogen and water vapor has a total pressure of 2.015 atm. The partial pressure of N2 is 1.908 atm. Calculate the partial pressure of the water vapor.
A) 0.107 atm
B) 1.908 atm
C) 2.015 atm
D) 3.923 atm
A) 0.107 atm
B) 1.908 atm
C) 2.015 atm
D) 3.923 atm

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
72
Dry air is 78.08% nitrogen, 20.95% oxygen, and 0.93% argon with the 0.04% consisting of other gases. Calculate the partial pressure of oxygen in dry air when the atmospheric pressure is 760 torr.
A) 20.95 torr
B) 159.2 torr
C) 600.8 torr
D) 760.0 torr
A) 20.95 torr
B) 159.2 torr
C) 600.8 torr
D) 760.0 torr

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
73
A tank contains N2 at 2.5 atm and O2 at 1.5 atm. An unknown quantity of CO2 is added until the total pressure within the tank is 5.5 atm. Calculate the partial pressure of CO2 in the tank.
A) 5.5 atm
B) 3.5 atm
C) 1.5 atm
D) 2.5 atm
A) 5.5 atm
B) 3.5 atm
C) 1.5 atm
D) 2.5 atm

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following is true of hydrogen bonding?
A) Compounds that contain H-O, H-F, or H-N bonds show hydrogen bonding.
B) Compounds that contain H-S, H-Cl, or H-P bonds show hydrogen bonding.
C) Both of these are true.
D) None of these are true.
A) Compounds that contain H-O, H-F, or H-N bonds show hydrogen bonding.
B) Compounds that contain H-S, H-Cl, or H-P bonds show hydrogen bonding.
C) Both of these are true.
D) None of these are true.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
75
Which type of intermolecular attraction exists between molecules?
A) Covalent bonding
B) Dipole-dipole interactions
C) London dispersion forces
D) Hydrogen bonding
A) Covalent bonding
B) Dipole-dipole interactions
C) London dispersion forces
D) Hydrogen bonding

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
76
A vessel containing CO2 and H2O has a pressure of 2.502 atm. The partial pressure of CO2 is 1.980 atm. Calculate the partial pressure of H2O in the vessel.
A) 0.522 atm
B) 0.955 atm
C) 1.204 atm
D) 4.482 atm
A) 0.522 atm
B) 0.955 atm
C) 1.204 atm
D) 4.482 atm

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
77
What is solidification?
A) It is the change of a substance from the liquid state to the solid state.
B) It is the change of a substance from the gaseous state to the liquid state.
C) It is the change of a substance from the liquid state to the gaseous state.
D) It is the change of a substance from the solid state to the liquid state.
A) It is the change of a substance from the liquid state to the solid state.
B) It is the change of a substance from the gaseous state to the liquid state.
C) It is the change of a substance from the liquid state to the gaseous state.
D) It is the change of a substance from the solid state to the liquid state.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
78
A closed flask contains 0.25 mol of O2 that exerts a pressure of 0.50 atm. Calculate the partial pressure of oxygen when 0.75 mol of CO2 is added to the container.
A) 0.50 atm
B) 1.0 atm
C) 1.5 atm
D) 2.0 atm
A) 0.50 atm
B) 1.0 atm
C) 1.5 atm
D) 2.0 atm

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following influences the strength of London dispersion forces?
A) the number of electrons
B) the size of molecules
C) the shape of molecules
D) all of these
A) the number of electrons
B) the size of molecules
C) the shape of molecules
D) all of these

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following is the strongest intermolecular interaction?
A) Covalent bonding
B) Dipole-dipole interactions
C) London dispersion forces
D) Hydrogen bonding
A) Covalent bonding
B) Dipole-dipole interactions
C) London dispersion forces
D) Hydrogen bonding

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck


