Exam 5: Gases, Liquids, and Solids
Exam 1: Matter, Energy, and Measurement143 Questions
Exam 2: Atoms134 Questions
Exam 3: Chemical Bonds142 Questions
Exam 4: Chemical Reactions138 Questions
Exam 5: Gases, Liquids, and Solids104 Questions
Exam 6: Solutions and Colloids157 Questions
Exam 7: Reaction Rates and Chemical Equilibrium104 Questions
Exam 8: Acids and Bases198 Questions
Exam 9: Nuclear Chemistry152 Questions
Exam 10: Organic Chemistry71 Questions
Exam 11: Alkanes142 Questions
Exam 12: Alkenes, Alkynes, and Aromatic Compounds184 Questions
Exam 13: Alcohols, Ethers, and Thiols118 Questions
Exam 14: Chirality: the Handedness of Molecules92 Questions
Exam 15: Amines89 Questions
Exam 16: Aldehydes and Ketones102 Questions
Exam 17: Carboxylic Acids115 Questions
Exam 18: Carboxylic Anhydrides, Esters, and Amides117 Questions
Exam 19: Carbohydrates103 Questions
Exam 20: Lipids132 Questions
Exam 21: Proteins128 Questions
Exam 22: Enzymes62 Questions
Exam 23: Chemical Communications: Neurotransmitters and Hormones89 Questions
Exam 24: Nucleotides, Nucleic Acids, and Heredity121 Questions
Exam 25: Gene Expression and Protein Synthesis129 Questions
Exam 26: Bioenergetics: How the Body Converts Food to Energy133 Questions
Exam 27: Specific Catabolic Pathways: Carbohydrate, Lipid, and Protein Metabolism104 Questions
Exam 28: Biosynthetic Pathways67 Questions
Exam 29: Nutrition73 Questions
Exam 30: Immunochemistry132 Questions
Exam 31: Body Fluids72 Questions
Select questions type
Which of the following best describes the relationship of molecules in a liquid with those in a gas at the same temperature?
Free
(Multiple Choice)
4.9/5  (36)
(36)
Correct Answer:
C
Which of the following relates to the term "vapor pressure of a liquid"?
Free
(Multiple Choice)
4.7/5  (39)
(39)
Correct Answer:
B
When the state of a substance changes from A to B as represented by the given models, which of the following statements is true? 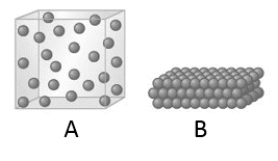
Free
(Multiple Choice)
4.9/5  (32)
(32)
Correct Answer:
C
Which of the following is the strongest intermolecular interaction?
(Multiple Choice)
4.7/5  (33)
(33)
Which of the following is a mathematical representation of the combined gas law?
(Multiple Choice)
4.9/5  (40)
(40)
The normal boiling point of acetic acid is 117.9°C. Which of the following is true?
(Multiple Choice)
4.8/5  (26)
(26)
Which of the following is a mathematical representation of Charles's law?
(Multiple Choice)
4.7/5  (36)
(36)
An unknown amount of He gas occupies 30.5 L at 2.00 atm pressure and 300 K. Calculate the weight of the gas in the container.
(Multiple Choice)
4.7/5  (30)
(30)
At constant pressure, the temperature of a 4.00 L sample of gas is increased from 300 K to 400 K. Calculate the new volume of the gas sample.
(Multiple Choice)
4.7/5  (41)
(41)
What is the average pressure of the atmosphere at sea level?
(Multiple Choice)
4.9/5  (31)
(31)
Which of the following leads to an increase in the kinetic energy of a molecule?
(Multiple Choice)
4.7/5  (28)
(28)
Suppose a balloon is filled so that its volume is 2.00 L when the pressure is 1.10 atm and the temperature is 300 K. What is the volume of the balloon when it rises to an elevation where the pressure is 418 mm Hg and the temperature is 200 K?
(Multiple Choice)
4.8/5  (34)
(34)
Chloroform has a normal boiling point of 61.7°C. Which of the following is true?
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following is a mathematical representation of the ideal gas law?
(Multiple Choice)
4.8/5  (25)
(25)
Which of the following is a mathematical representation of Boyle's law?
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following units can be used to measure air pressure?
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following is a mathematical representation of Gay-Lussac's law?
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following are measures of standard temperature and pressure?
(Multiple Choice)
4.8/5  (41)
(41)
At constant pressure, the temperature of a 4.0 L sample of gas is decreased from 50°C to 25°C. Calculate the new volume of the gas sample.
(Multiple Choice)
5.0/5  (30)
(30)
Showing 1 - 20 of 104
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)