Deck 5: Aromatic Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/52
Play
Full screen (f)
Deck 5: Aromatic Compounds
1
Circle the compound(s) in the following set that will undergo both substitution and addition when treated with Br2. 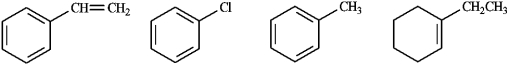

2
Which of the following is a correct statement regarding electrophilic aromatic substitution?
A) The carbocation intermediate will lose a proton to regain aromaticity, usually from a position other than the site of electrophilic attack.
B) Formation of the carbocation intermediate has a high activation barrier due to loss of aromaticity.
C) The carbocation intermediate has several resonance structures and is negatively charged.
D) Re-formation of the aromatic ring has a low activation barrier and therefore occurs slowly.
E) Many suitable electrophiles are unreactive and can be stored for long periods of time prior to use.
A) The carbocation intermediate will lose a proton to regain aromaticity, usually from a position other than the site of electrophilic attack.
B) Formation of the carbocation intermediate has a high activation barrier due to loss of aromaticity.
C) The carbocation intermediate has several resonance structures and is negatively charged.
D) Re-formation of the aromatic ring has a low activation barrier and therefore occurs slowly.
E) Many suitable electrophiles are unreactive and can be stored for long periods of time prior to use.
Formation of the carbocation intermediate has a high activation barrier due to loss of aromaticity.
3
Which of the following undergoes the most rapid bromination upon treatment with Br2/FeBr3?
A) benzene
B) nitrobenzene
C) bromobenzene
D) benzaldehyde
A) benzene
B) nitrobenzene
C) bromobenzene
D) benzaldehyde
benzene
4
Which of the following compounds is aromatic?
A) ethane
B) cyclobuta-1,3-diene
C) benzene
D) cycloocta-1,3,5,7-tetraene
A) ethane
B) cyclobuta-1,3-diene
C) benzene
D) cycloocta-1,3,5,7-tetraene

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
5
Consider the following sequence of reactions: 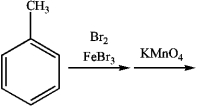
1)What is the major organic product obtained from the sequence of reactions?
A)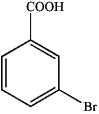
B)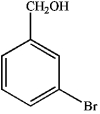
C)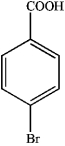
D)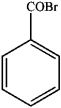
2)If KMnO4 had been replaced by Na2Cr2O7, what would be the final product of the reaction?

1)What is the major organic product obtained from the sequence of reactions?
A)

B)

C)

D)

2)If KMnO4 had been replaced by Na2Cr2O7, what would be the final product of the reaction?

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following sets of substituents are all deactivating groups in electrophilic aromatic substitution reactions?
A) Cl, CN, NO2
B) Cl, NH2, CH3
C) CH3, OCH3, COCH3
D) CH3, NH2, OCH3
A) Cl, CN, NO2
B) Cl, NH2, CH3
C) CH3, OCH3, COCH3
D) CH3, NH2, OCH3

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
7
Consider the following compound: 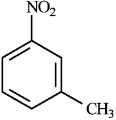 a)Provide the correct IUPAC name for the compound.
a)Provide the correct IUPAC name for the compound.
b)Draw and name the other isomeric nitrotoluenes.
 a)Provide the correct IUPAC name for the compound.
a)Provide the correct IUPAC name for the compound.b)Draw and name the other isomeric nitrotoluenes.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
8
Draw the resonance structures for acenaphthene. 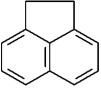


Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
9
What is the correct assignment of the names of the following aromatic compounds? 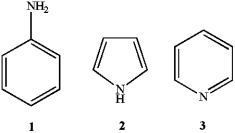
A) 1 = anisole; 2 = furan; 3 = pyrimidine
B) 1 = aniline; 2 = pyrrole; 3 = pyridine
C) 1 = anisole; 2 = pyridine; 3 = pyrrole
D) 1 = aniline; 2 = imidazole; 3 = pyridine

A) 1 = anisole; 2 = furan; 3 = pyrimidine
B) 1 = aniline; 2 = pyrrole; 3 = pyridine
C) 1 = anisole; 2 = pyridine; 3 = pyrrole
D) 1 = aniline; 2 = imidazole; 3 = pyridine

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
10
Draw the structure of 4-bromo-2-fluorotoluene.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
11
Place the following in order of reactivity towards electrophilic aromatic substitution. 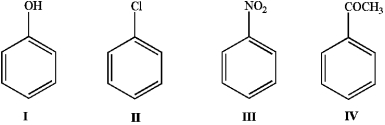
A) I > II > III > IV
B) I > II > IV > III
C) II > I > III > IV
D) II > I > IV > III
E) III > IV > II > I

A) I > II > III > IV
B) I > II > IV > III
C) II > I > III > IV
D) II > I > IV > III
E) III > IV > II > I

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
12
An accurate description of the structure of benzene is:
A) The bonds are quickly moving around the ring.
B) There are two distinct structures that are in equilibrium.
C) All the carbon-carbon bonds are equal in length.
D) There are distinct single and double bonds.
E) Some bonds are longer than others.
A) The bonds are quickly moving around the ring.
B) There are two distinct structures that are in equilibrium.
C) All the carbon-carbon bonds are equal in length.
D) There are distinct single and double bonds.
E) Some bonds are longer than others.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following undergoes the most rapid acylation upon treatment with acetyl chloride and AlCl3?
A) benzene
B) toluene
C) chlorobenzene
D) 1,4-dichlorobenzene
A) benzene
B) toluene
C) chlorobenzene
D) 1,4-dichlorobenzene

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following sets of substituents are all o/p-directing in electrophilic aromatic substitution reactions?
A) Cl, CH3, CN
B) Br, OH, COCH3
C) Cl, OH, CH3
D) CN, NO2, COCH3
A) Cl, CH3, CN
B) Br, OH, COCH3
C) Cl, OH, CH3
D) CN, NO2, COCH3

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
15
What is the IUPAC name of the following compound? 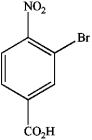
A) 3-bromo-4-nitrobenzaldehyde
B) 2-bromo-1-nitro-4-benzoic acid
C) 3-bromo-4-nitroacetophenone
D) 3-bromo-4-nitrobenzoic acid

A) 3-bromo-4-nitrobenzaldehyde
B) 2-bromo-1-nitro-4-benzoic acid
C) 3-bromo-4-nitroacetophenone
D) 3-bromo-4-nitrobenzoic acid

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
16
What is the major organic product obtained from the following reaction? 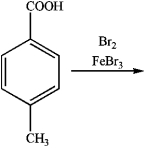
A)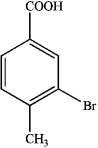
B)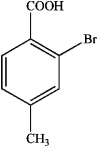
C)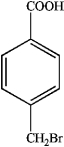
D)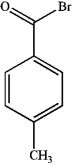

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
17
Draw the major product of the following reaction: 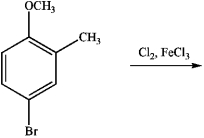


Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
18
Consider the production of 1-bromo-4-nitrobenzene: 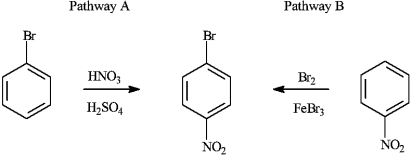
a)Explain why Pathway A for the production of 1-bromo-4-nitrobenzene produces a higher yield of the product.
b)If reaction Pathway A is carried out at high temperatures, a trisubstituted product forms. Draw the structure of the product and explain why it forms.

a)Explain why Pathway A for the production of 1-bromo-4-nitrobenzene produces a higher yield of the product.
b)If reaction Pathway A is carried out at high temperatures, a trisubstituted product forms. Draw the structure of the product and explain why it forms.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
19
What is the IUPAC name of the following compound? 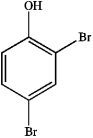
A) 2,4-dibromotoluene
B) 2,4-dibromophenol
C) 2,4-dibromoaniline
D) 4,6-dibromophenol

A) 2,4-dibromotoluene
B) 2,4-dibromophenol
C) 2,4-dibromoaniline
D) 4,6-dibromophenol

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
20
What is the major organic product obtained from the following reaction? 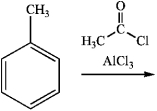
A)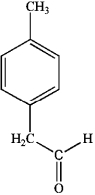
B)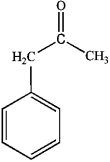
C)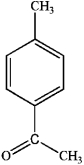
D)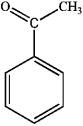

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
21
Instructions: Provide the IUPAC name for each of the following compounds.
IUPAC Name: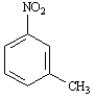
IUPAC Name:


Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
22
Instructions: Provide the IUPAC name for each of the following compounds.
IUPAC Name: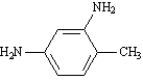
IUPAC Name:


Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
23
Instructions: Consider the reaction below to answer the following question(s).
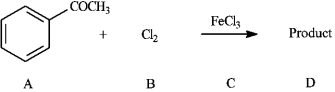
Refer to instructions. This reaction proceeds _____________ (faster or slower) than benzene.

Refer to instructions. This reaction proceeds _____________ (faster or slower) than benzene.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
24
On the structures provided below, draw arrows showing the complete stepwise mechanism for this reaction. 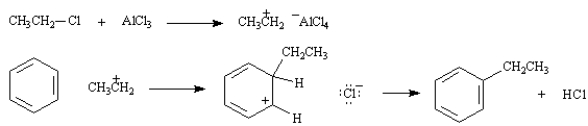


Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
25
Draw the structure of p-chlorobenzaldehyde.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
26
Instructions: Consider the reaction below to answer the following question(s).

Refer to instructions. The Lewis acid catalyst in the reaction is indicated by letter _____.

Refer to instructions. The Lewis acid catalyst in the reaction is indicated by letter _____.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
27
Instructions: Consider the reaction below to answer the following question(s).

Refer to instructions. Draw the structure of product D.

Refer to instructions. Draw the structure of product D.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
28
Instructions:
Consider the Friedel-Crafts alkylation reaction below to answer the following questions: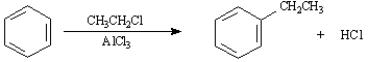
What is the role of the AlCl3 in the reaction?
Consider the Friedel-Crafts alkylation reaction below to answer the following questions:

What is the role of the AlCl3 in the reaction?

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
29
Draw the structure of p-bromoaniline.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
30
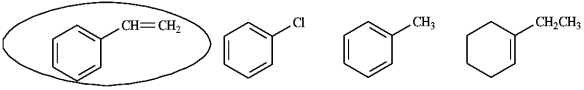
Circle the aromatic portion of the following molecule: 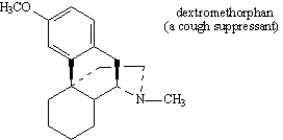


Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
31
Draw the structure of m-fluoronitrobenzene.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following heterocycles is aromatic?
A)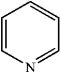
B)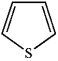
C)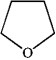
D)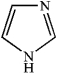
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
33
Instructions: Consider the reaction below to answer the following question(s).

Refer to instructions. The nucleophile in the reaction is indicated by letter _____.

Refer to instructions. The nucleophile in the reaction is indicated by letter _____.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
34
Draw the structure of 3,5-dimethylphenol.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
35
Instructions:
Consider the Friedel-Crafts alkylation reaction below to answer the following questions:
Draw the structure of the electrophile in this reaction.
Consider the Friedel-Crafts alkylation reaction below to answer the following questions:

Draw the structure of the electrophile in this reaction.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
36
Draw the structure of o-hydroxybenzoic acid.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
37
Circle the aromatic portion of the following molecule: 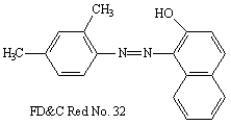


Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
38
Instructions: Provide the IUPAC name for each of the following compounds.
IUPAC Name: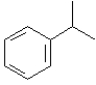
IUPAC Name:


Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
39
Instructions: Provide the IUPAC name for each of the following compounds.
IUPAC Name: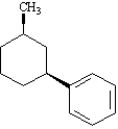
IUPAC Name:


Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
40
Instructions: Provide the IUPAC name for each of the following compounds.
IUPAC Name: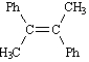
IUPAC Name:


Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
41
Instructions:
Choose the best reagent(s) from the list provided below for carrying out the following conversions. Place the letter of the reagent in the box beside the reaction number over the arrow. There is only one answer for each reaction.
a. KMnO4, H3O+
b. Br2, FeBr3
c. Cl2, FeCl3
d. CH3Cl, AlCl3
e. HNO3, H2SO4
Choose the best reagents: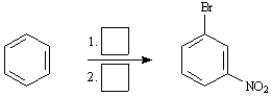
Choose the best reagent(s) from the list provided below for carrying out the following conversions. Place the letter of the reagent in the box beside the reaction number over the arrow. There is only one answer for each reaction.
a. KMnO4, H3O+
b. Br2, FeBr3
c. Cl2, FeCl3
d. CH3Cl, AlCl3
e. HNO3, H2SO4
Choose the best reagents:


Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
42
Instructions:
Given the major organic product(s) of each of the following reactions. If no reaction is predicted, write "N.R."
Write the product: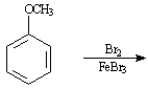
Given the major organic product(s) of each of the following reactions. If no reaction is predicted, write "N.R."
Write the product:


Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
43
Instructions:
Given the major organic product(s) of each of the following reactions. If no reaction is predicted, write "N.R."
Write the product: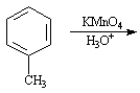
Given the major organic product(s) of each of the following reactions. If no reaction is predicted, write "N.R."
Write the product:


Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
44
Instructions:
Given the major organic product(s) of each of the following reactions. If no reaction is predicted, write "N.R."
Write the product: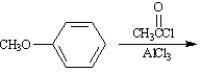
Given the major organic product(s) of each of the following reactions. If no reaction is predicted, write "N.R."
Write the product:
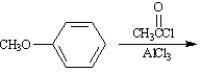

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
45
Instructions:
Starting with benzene or toluene, how would you synthesize these compounds?
Assume ortho and para isomers can be separated.
Synthesize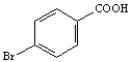
Starting with benzene or toluene, how would you synthesize these compounds?
Assume ortho and para isomers can be separated.
Synthesize


Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
46
Instructions:
Choose the best reagent(s) from the list provided below for carrying out the following conversions. Place the letter of the reagent in the box beside the reaction number over the arrow. There is only one answer for each reaction.
a. KMnO4, H3O+
b. Br2, FeBr3
c. Cl2, FeCl3
d. CH3Cl, AlCl3
e. HNO3, H2SO4
Choose the best reagents: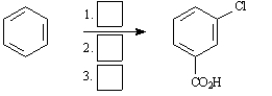
Choose the best reagent(s) from the list provided below for carrying out the following conversions. Place the letter of the reagent in the box beside the reaction number over the arrow. There is only one answer for each reaction.
a. KMnO4, H3O+
b. Br2, FeBr3
c. Cl2, FeCl3
d. CH3Cl, AlCl3
e. HNO3, H2SO4
Choose the best reagents:


Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
47
Instructions:
Starting with benzene or toluene, how would you synthesize these compounds?
Assume ortho and para isomers can be separated.
Synthesize: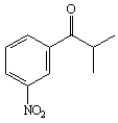
Starting with benzene or toluene, how would you synthesize these compounds?
Assume ortho and para isomers can be separated.
Synthesize:


Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
48
Instructions:
Given the major organic product(s) of each of the following reactions. If no reaction is predicted, write "N.R."
Write the product: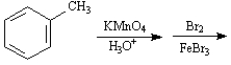
Given the major organic product(s) of each of the following reactions. If no reaction is predicted, write "N.R."
Write the product:
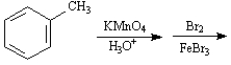

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
49
Instructions:
Given the major organic product(s) of each of the following reactions. If no reaction is predicted, write "N.R."
Write the product: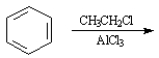
Given the major organic product(s) of each of the following reactions. If no reaction is predicted, write "N.R."
Write the product:
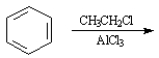

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
50
Instructions:
Given the major organic product(s) of each of the following reactions. If no reaction is predicted, write "N.R."
Write the product: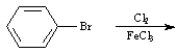
Given the major organic product(s) of each of the following reactions. If no reaction is predicted, write "N.R."
Write the product:
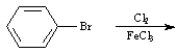

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
51
Instructions:
Given the major organic product(s) of each of the following reactions. If no reaction is predicted, write "N.R."
Write the product: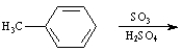
Given the major organic product(s) of each of the following reactions. If no reaction is predicted, write "N.R."
Write the product:


Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
52
Instructions:
Given the major organic product(s) of each of the following reactions. If no reaction is predicted, write "N.R."
Write the product: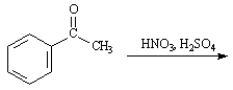
Given the major organic product(s) of each of the following reactions. If no reaction is predicted, write "N.R."
Write the product:


Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck


