Exam 5: Aromatic Compounds
Exam 1: Structure and Bonding:acids and Bases41 Questions
Exam 2: Alkanes: the Nature of Organic Compounds44 Questions
Exam 3: Alkenes and Alkynes: the Nature of Organic Reactions40 Questions
Exam 4: Reactions of Alkenes and Alkynes44 Questions
Exam 5: Aromatic Compounds52 Questions
Exam 6: Sterechemistry at Tetrahedral Centers39 Questions
Exam 7: Organohalides: Nucleophilic Substitutions and Eliminations40 Questions
Exam 8: Alcohols, Phenols, Ethers, and Their Sulfur Analogs36 Questions
Exam 9: Aldehydes and Ketones: Nucleophilic Addition Reactions32 Questions
Exam 10: Carboxylic Acids and Derivatives: Nucleophilic Acyl Substitution Reactions63 Questions
Exam 11: Carbonyl Alpha-Substitution Reactions and Condensation Reactions38 Questions
Exam 12: Amines32 Questions
Exam 13: Structure Determination65 Questions
Exam 14: Biomolecules: Carbohydrates48 Questions
Exam 15: Biomolecules: Amino Acids, Peptides, and Proteins50 Questions
Exam 16: Biomolecules: Lipids and Nucleic Acids50 Questions
Exam 17: The Organic Chemistry of Metabolic Pathways40 Questions
Select questions type
What is the IUPAC name of the following compound? 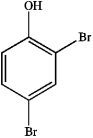
Free
(Multiple Choice)
4.9/5  (36)
(36)
Correct Answer:
B
Instructions: Consider the reaction below to answer the following question(s).
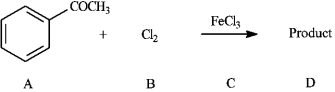 -Refer to instructions. The Lewis acid catalyst in the reaction is indicated by letter _____.
-Refer to instructions. The Lewis acid catalyst in the reaction is indicated by letter _____.
Free
(Short Answer)
4.8/5  (35)
(35)
Correct Answer:
C
Draw the major product of the following reaction: 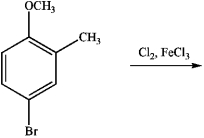
Free
(Essay)
4.8/5  (37)
(37)
Correct Answer:
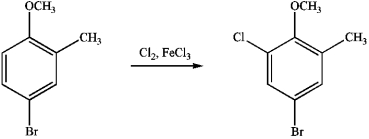
Instructions:
Given the major organic product(s) of each of the following reactions. If no reaction is predicted, write "N.R."
-Write the product: 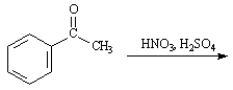
(Essay)
4.9/5  (31)
(31)
Place the following in order of reactivity towards electrophilic aromatic substitution. 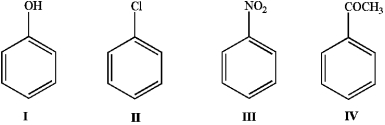
(Multiple Choice)
4.8/5  (46)
(46)
Instructions:
Given the major organic product(s) of each of the following reactions. If no reaction is predicted, write "N.R."
-Write the product: 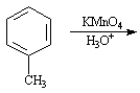
(Essay)
4.8/5  (29)
(29)
Instructions:
Starting with benzene or toluene, how would you synthesize these compounds?
Assume ortho and para isomers can be separated.
-Synthesize 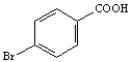
(Essay)
4.7/5  (35)
(35)
Instructions: Provide the IUPAC name for each of the following compounds.
-IUPAC Name: 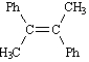
(Essay)
4.8/5  (33)
(33)
Instructions:
Given the major organic product(s) of each of the following reactions. If no reaction is predicted, write "N.R."
-Write the product: 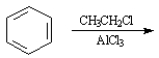
(Essay)
4.9/5  (46)
(46)
Instructions: Consider the reaction below to answer the following question(s).
 -Refer to instructions. Draw the structure of product D.
-Refer to instructions. Draw the structure of product D.
(Essay)
4.8/5  (31)
(31)
Which of the following undergoes the most rapid bromination upon treatment with Br2/FeBr3?
(Multiple Choice)
4.8/5  (39)
(39)
Instructions:
Choose the best reagent(s) from the list provided below for carrying out the following conversions. Place the letter of the reagent in the box beside the reaction number over the arrow. There is only one answer for each reaction.
a. KMnO4, H3O+
b. Br2, FeBr3
c. Cl2, FeCl3
d. CH3Cl, AlCl3
e. HNO3, H2SO4
-Choose the best reagents: 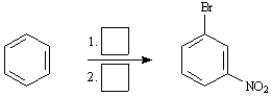
(Essay)
4.8/5  (38)
(38)
Circle the compound(s) in the following set that will undergo both substitution and addition when treated with Br2. 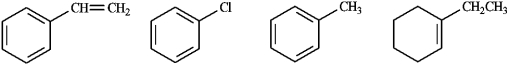
(Essay)
4.8/5  (38)
(38)
Instructions:
Given the major organic product(s) of each of the following reactions. If no reaction is predicted, write "N.R."
-Write the product: 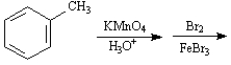
(Essay)
4.7/5  (35)
(35)
Instructions:
Consider the Friedel-Crafts alkylation reaction below to answer the following questions: 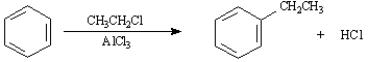 -What is the role of the AlCl3 in the reaction?
-What is the role of the AlCl3 in the reaction?
(Essay)
4.8/5  (46)
(46)
Instructions:
Given the major organic product(s) of each of the following reactions. If no reaction is predicted, write "N.R."
-Write the product: 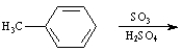
(Essay)
4.8/5  (38)
(38)
Instructions: Provide the IUPAC name for each of the following compounds.
-IUPAC Name: 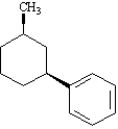
(Short Answer)
4.9/5  (41)
(41)
Showing 1 - 20 of 52
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)