Deck 16: Reactions Between Acids and Bases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/98
Play
Full screen (f)
Deck 16: Reactions Between Acids and Bases
1
In the titration of 0.100 M HCl with the titrant 0.100 M NaOH, what species are present at any point after NaOH has been added but before the equivalence point?
A) HCl only
B) NaOH only
C) NaCl only
D) HCl and NaOH
E) HCl and NaCl
A) HCl only
B) NaOH only
C) NaCl only
D) HCl and NaOH
E) HCl and NaCl
HCl and NaCl
2
In the titration of 0.100 M HCl with the titrant 0.100 M NaOH, what species are present at the equivalence point?
A) HCl only
B) NaOH only
C) NaCl only
D) NaOH and NaCl
E) HCl and NaCl
A) HCl only
B) NaOH only
C) NaCl only
D) NaOH and NaCl
E) HCl and NaCl
NaCl only
3
Calculate the pH of a solution made by mixing 20.00 mL of 0.300 M HCl and 50.00 mL of 0.100 M NaOH and diluting to a final volume of 1.00 L.
A) 3.00
B) 2.22
C) 2.30
D) - 1.18
E) 0.52
A) 3.00
B) 2.22
C) 2.30
D) - 1.18
E) 0.52
3.00
4
Calculate the volume of 0.100 M HCl required to neutralize 1.00 g of Ba(OH)2 (molar mass = 171.3 g/mol).
A) 58.4 mL
B) 117 mL
C) 29.2 mL
D) 585 mL
E) no reaction occurs
A) 58.4 mL
B) 117 mL
C) 29.2 mL
D) 585 mL
E) no reaction occurs

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
5
Calculate the pH of a solution after 10.0 mL of 0.100 M NaOH is added tO40.0 mL of 0.250 M HBr.
A) 0.65
B) 1.00
C) - 1.0
D) 13.01
E) 0.74
A) 0.65
B) 1.00
C) - 1.0
D) 13.01
E) 0.74

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
6
A 25.0 mL portion of 0.0500 M HCl was added to 10.0 mL of 0.0250 M NaOH. Calculate the pH of the resulting solution.
A) 12.54
B) 1.54
C) 3.00
D) 12.06
E) 1.30
A) 12.54
B) 1.54
C) 3.00
D) 12.06
E) 1.30

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
7
What volume of 0.200 M H₂ SO₄is required to neutralize 50.0 mL of 0.100 M NaOH?
Assume the chemical equation is:
H₂ SO₄(aq) + 2 NaOH (aq) Na2 SO₄(aq) + 2 H₂ O (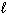 )
)
A) 50.0 mL
B) 25.0 mL
C) 100 mL
D) 12.5 mL
E) 6.25 mL
Assume the chemical equation is:
H₂ SO₄(aq) + 2 NaOH (aq) Na2 SO₄(aq) + 2 H₂ O (
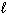 )
)A) 50.0 mL
B) 25.0 mL
C) 100 mL
D) 12.5 mL
E) 6.25 mL

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
8
Solutions are made by combining equal amounts of the following. Which is a buffer?
A) 0.1 M HCl + 0.1 M Cl -
B) 0.1 M HF + 0.1 M Na+
C) 0.1 M HF + 0.1 M NaOH
D) 0.1 M NaF + 0.05 M HF
E) 0.1 M NaF + 0.05 M Na+
A) 0.1 M HCl + 0.1 M Cl -
B) 0.1 M HF + 0.1 M Na+
C) 0.1 M HF + 0.1 M NaOH
D) 0.1 M NaF + 0.05 M HF
E) 0.1 M NaF + 0.05 M Na+

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
9
What is the molarity of Ba(OH)2 solution if 65.0 mL of 0.0500 M HCl is required to titrate 35.00 mL of the Ba(OH)2 solution?
A) 3.25×10 - 3 M
B) 9.28×10 - 2 M
C) 4.64×10 - 2 M
D) 2.69×10 - 3 M
E) 1.69×10 - 2 M
A) 3.25×10 - 3 M
B) 9.28×10 - 2 M
C) 4.64×10 - 2 M
D) 2.69×10 - 3 M
E) 1.69×10 - 2 M

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
10
In the titration of 50.0 mL of a 0.100 M HCl solution with 0.100 M NaOH, what is the pH of the solution after the addition of 20.0 mL of the NaOH solution?
A) 1.00
B) 1.22
C) 1.15
D) 1.37
E) 12.63
A) 1.00
B) 1.22
C) 1.15
D) 1.37
E) 12.63

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
11
Which pair(s) of substances would make a suitable buffer pair combination?
I. NaOH and NaBr
II. HF and NaF
III. CH3NH3+Cl - and CH3NH2
A) I only
B) II only
C) III only
D) I and II
E) II and III
I. NaOH and NaBr
II. HF and NaF
III. CH3NH3+Cl - and CH3NH2
A) I only
B) II only
C) III only
D) I and II
E) II and III

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
12
Calculate the pH of a solution formed when 1.5 L oF4.5×10 - 3 M HCl is mixed witH₂ .0 L of 3.0×10 - 3 M HNO3.
A) 3.67
B) 3.74
C) 2.25
D) 3.16
E) 2.44
A) 3.67
B) 3.74
C) 2.25
D) 3.16
E) 2.44

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following general combinations of substances form an acid-base buffer when added to water?
A) a strong acid and the sodium salt of its conjugate base
B) a strong base and the chloride salt of its conjugate acid
C) a weak acid and the sodium salt of its conjugate base
D) a weak acid
E) a weak base and a strong base
A) a strong acid and the sodium salt of its conjugate base
B) a strong base and the chloride salt of its conjugate acid
C) a weak acid and the sodium salt of its conjugate base
D) a weak acid
E) a weak base and a strong base

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
14
During a titration, 60.0 mL of 0.010 M NaOH is added to 35.0 mL of 0.020 M HCl solution. What is the pH of the resulting solution?
A) 1.70
B) 12.00
C) 2.98
D) 5.41
E) 4.05
A) 1.70
B) 12.00
C) 2.98
D) 5.41
E) 4.05

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
15
If it takes 32.0 mL of 0.100 M HCl to titrate 25.0 mL of a Ba(OH)2 solution to the equivalence point, what is the molarity of the original Ba(OH)2 solution?
A) 0.128 M
B) 3.20×10 - 2 M
C) 0.256 M
D) 6.40×10 - 2 M
E) 0.100 M
A) 0.128 M
B) 3.20×10 - 2 M
C) 0.256 M
D) 6.40×10 - 2 M
E) 0.100 M

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
16
A 350 mL volume of 0.150 M Ba(OH)2 was completely neutralized by 35.0 mL of HCl. What is the molarity of the HCl solution?
A) 1.50 M
B) 3.00 M
C) 0.750 M
D) 1.00 M
E) 0.500 M
A) 1.50 M
B) 3.00 M
C) 0.750 M
D) 1.00 M
E) 0.500 M

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
17
What is the pH of a solution of 25.0 mL of 0.250 M HNO3 after the addition oF40.0 mL of 0.300 M KOH?
A) 1.02
B) 0.60
C) 12.95
D) 13.25
E) 13.45
A) 1.02
B) 0.60
C) 12.95
D) 13.25
E) 13.45

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
18
In the titration of 0.100 M HCl with the titrant 0.100 M NaOH, what species are present after the equivalence point?
A) HCl only
B) NaOH only
C) NaCl only
D) NaOH and NaCl
E) HCl and NaCl
A) HCl only
B) NaOH only
C) NaCl only
D) NaOH and NaCl
E) HCl and NaCl

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
19
A 0.40 gram sample of NaOH (molar mass = 40.0 g/mol) was completely neutralized witH₆2.5 mL of H₂ SO₄solution. Assume the chemical equation is:
H₂ SO₄(aq) + 2 NaOH (aq) Na2 SO₄(aq) + 2 H₂ O (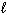 ) The molarity of the H₂ SO₄solution was:
) The molarity of the H₂ SO₄solution was:
A) 0.080 M
B) 0.16 M
C) 0.62 M
D) 6.4 M
E) 3.2 M
H₂ SO₄(aq) + 2 NaOH (aq) Na2 SO₄(aq) + 2 H₂ O (
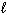 ) The molarity of the H₂ SO₄solution was:
) The molarity of the H₂ SO₄solution was:A) 0.080 M
B) 0.16 M
C) 0.62 M
D) 6.4 M
E) 3.2 M

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
20
What is the volume of 0.0175 M Ba(OH)2 required to neutralize 10.0 mL of 0.0300 M HCl?
A) 10.0 mL
B) 34.2 mL
C) 17.1 mL
D) 8.6 mL
E) 4.3 mL
A) 10.0 mL
B) 34.2 mL
C) 17.1 mL
D) 8.6 mL
E) 4.3 mL

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
21
Which one of these combinations would give a buffer that would be most effective in the pH range 3.0 tO4.0?
A) 0.10 M CH3COOH, 0.10 M CH3COONa, K a = 1.8×10 - 5
B) 0.10 M NH4Cl, 0.10 M NH3, K b = 1.8×10 - 5
C) 0.10 M HF, 0.10 M NaF, K a = 3.54×10 - 4
D) 0.10 M H2CO3, 0.10 M NaHCO3, K a = 4.30×10 - 7
E) 0.10 M HCl, 0.10 NaCl
A) 0.10 M CH3COOH, 0.10 M CH3COONa, K a = 1.8×10 - 5
B) 0.10 M NH4Cl, 0.10 M NH3, K b = 1.8×10 - 5
C) 0.10 M HF, 0.10 M NaF, K a = 3.54×10 - 4
D) 0.10 M H2CO3, 0.10 M NaHCO3, K a = 4.30×10 - 7
E) 0.10 M HCl, 0.10 NaCl

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
22
Consider a buffer solution containing a weak acid, HX, and a salt of the weak acid's conjugate base, NaX. Under what conditions is the pH greater than the p K a of the weak acid?
A) When the p K a equals 7.0.
B) When [HX] > [NaX].
C) When [HX] < [NaX].
D) When [HX] = [NaX].
E) When there is no NaX present.
A) When the p K a equals 7.0.
B) When [HX] > [NaX].
C) When [HX] < [NaX].
D) When [HX] = [NaX].
E) When there is no NaX present.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
23
What is the pH of a buffer solution that consists of 0.83 M HBrO and 0.53 M KBrO?
K a(HBrO) = 2.3×10 - 9
A) pH = 8.19
B) pH = 8.45
C) pH = 8.83
D) pH = 8.93
E) pH = 12.05
K a(HBrO) = 2.3×10 - 9
A) pH = 8.19
B) pH = 8.45
C) pH = 8.83
D) pH = 8.93
E) pH = 12.05

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
24
A solution of HCOOH ( K a = 1.8×10 - 4) and HCOO - was submitted for chemical analysis. The results were:
[HCOOH] = 0.050 M, [HCOO - ] = 0.15 M. Calculate the pH.
A) 4.22
B) 3.74
C) 3.27
D) 1.30
E) - 1.30
[HCOOH] = 0.050 M, [HCOO - ] = 0.15 M. Calculate the pH.
A) 4.22
B) 3.74
C) 3.27
D) 1.30
E) - 1.30

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
25
Consider the buffer pair, NH4Cl/NH3. What can be stated about the relative proportions of this buffer pair if the pH of an aqueous solution of this pair is adjusted to 12.00 and the p K a for NH4+ equals 9.25?
A) This buffer solution contains relatively more NH4+ than NH3.
B) This buffer solution contains relatively more NH3 than NH4+.
C) This buffer solution contains equal concentrations of NH4+ and NH3.
D) More information is needed to determine the relative proportion of each member.
A) This buffer solution contains relatively more NH4+ than NH3.
B) This buffer solution contains relatively more NH3 than NH4+.
C) This buffer solution contains equal concentrations of NH4+ and NH3.
D) More information is needed to determine the relative proportion of each member.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
26
Which pair(s) of substances would make a suitable buffer pair ?
I. HCl and NaCl
II. HF and NaF
III. NH4Cl and NH3
A) I only
B) II only
C) III only
D) I and II
E) II and III
I. HCl and NaCl
II. HF and NaF
III. NH4Cl and NH3
A) I only
B) II only
C) III only
D) I and II
E) II and III

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
27
A buffer solution maintains a constant pH level to a certain extent when a strong base such as NaOH is added. Which of the following buffer solutions has the greatest buffering capacity to consume added NaOH?
A) 0.5 M HF/1.0 M NaF
B) 0.5 M HF/1.5 M NaF
C) 1.0 M HF/0.5 M NaF
D) 1.0 M HF/1.5 M NaF
E) 1.5 M HF/0.5 M NaF
A) 0.5 M HF/1.0 M NaF
B) 0.5 M HF/1.5 M NaF
C) 1.0 M HF/0.5 M NaF
D) 1.0 M HF/1.5 M NaF
E) 1.5 M HF/0.5 M NaF

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
28
What is the pH of a solution that is 0.100 M methylamine (CH3NH2) and 0.200 M methyl ammonium chloride (CH3NH3Cl)?
The K b of methylamine is 3.70×10 - 4.
A) 1.00
B) 3.73
C) 10.73
D) 3.43
E) 10.27
The K b of methylamine is 3.70×10 - 4.
A) 1.00
B) 3.73
C) 10.73
D) 3.43
E) 10.27

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following would not make a suitable buffer pair?
A) NH4Cl/NH3
B) HNO3/NaNO3
C) NaHCO3/Na2CO3
D) H3PO4/KH2PO4
E) HCN/NaCN
A) NH4Cl/NH3
B) HNO3/NaNO3
C) NaHCO3/Na2CO3
D) H3PO4/KH2PO4
E) HCN/NaCN

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
30
Consider the two buffer solutions prepared below:
Solution A:
[HA] = 0.15 M and [A - ] = 0.25 M Solution B:
[HA] = 1.50 M and [A - ] = 2.50 M Which statement below is true regarding the pH and buffering capacity of these two solutions?
A) Solution A will have a greater pH value and a smaller buffering capacity.
B) Solution B will have a greater pH value and a smaller buffering capacity.
C) Solution B will have a greater pH value and a larger buffering capacity.
D) The pH of both solutions will be identical but Solution A will have a larger buffering capacity.
E) The pH of both solutions will be identical but Solution B will have a larger buffering capacity.
Solution A:
[HA] = 0.15 M and [A - ] = 0.25 M Solution B:
[HA] = 1.50 M and [A - ] = 2.50 M Which statement below is true regarding the pH and buffering capacity of these two solutions?
A) Solution A will have a greater pH value and a smaller buffering capacity.
B) Solution B will have a greater pH value and a smaller buffering capacity.
C) Solution B will have a greater pH value and a larger buffering capacity.
D) The pH of both solutions will be identical but Solution A will have a larger buffering capacity.
E) The pH of both solutions will be identical but Solution B will have a larger buffering capacity.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
31
Consider a buffer solution containing a weak acid, HX, and a salt of the weak acid's conjugate base, NaX. Under what conditions does the pH just equal the p K a of the weak acid?
A) When the p K a equals 7.0.
B) When [HX] > [NaX].
C) When [HX] < [NaX].
D) When [HX] = [NaX].
E) When there is no NaX present.
A) When the p K a equals 7.0.
B) When [HX] > [NaX].
C) When [HX] < [NaX].
D) When [HX] = [NaX].
E) When there is no NaX present.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
32
Calculate the pH of a solution that is 0.78 M NH3 and 0.140 M NH4NO3. K b = 1.8×10 - 5 for NH3.
A) 4.00
B) 10.00
C) 4.74
D) 9.26
E) 13.91
A) 4.00
B) 10.00
C) 4.74
D) 9.26
E) 13.91

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
33
Calculate the pH of a buffer prepared by dissolving 0.10 mol of NH4Cl in 1.00 L of 0.15 M NH3. K b = 1.8×10 - 5 for NH3.
A) 9.43
B) 4.56
C) 11.34
D) 2.72
E) 4.74
A) 9.43
B) 4.56
C) 11.34
D) 2.72
E) 4.74

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
34
What is the pH of an aqueous solution that is 0.55 M HNO2 and 0.75 M KNO2?
K a(HNO2) = 7.1×10 - 4
A) pH = 3.01
B) pH = 3.28
C) pH = 3.63
D) pH = 6.94
E) pH = 7.56
K a(HNO2) = 7.1×10 - 4
A) pH = 3.01
B) pH = 3.28
C) pH = 3.63
D) pH = 6.94
E) pH = 7.56

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
35
What is the pH of an aqueous solution that contains 0.085 M HNO2 and 0.10 M potassium nitrite (KNO2)?
K a(HNO2) = 4.5×10 - 4
A) pH = 3.27
B) pH = 3.42
C) pH = 4.28
D) pH = 4.42
E) pH = 7.87
K a(HNO2) = 4.5×10 - 4
A) pH = 3.27
B) pH = 3.42
C) pH = 4.28
D) pH = 4.42
E) pH = 7.87

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
36
Consider the buffer pair, HF/F - . What can be stated about the relative proportions of this buffer pair if the pH of an aqueous solution of this pair is adjusted tO4.00 and the p K a for HF equals 3.17?
A) This buffer solution contains relatively more HF than F - .
B) This buffer solution contains relatively more F - than HF.
C) This buffer solution contains equal concentrations of HF and F - .
D) More information is needed to determine the relative proportion of each member.
A) This buffer solution contains relatively more HF than F - .
B) This buffer solution contains relatively more F - than HF.
C) This buffer solution contains equal concentrations of HF and F - .
D) More information is needed to determine the relative proportion of each member.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
37
A solution is made by dissolving 0.100 mole NH3 ( K b = 1.8×10 - 5) and 0.200 mole of NH4Cl in water and diluting to 1.00 L. What is the pH of the resulting solution?
A) 8.95
B) 11.12
C) 2.72
D) 13.00
E) 5.04
A) 8.95
B) 11.12
C) 2.72
D) 13.00
E) 5.04

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
38
What is the pH of a solution prepared by mixing 0.250 mol of hydrazoic acid, HN3, and 0.500 mol of sodium azide, NaN3, to make 1.00 liter of solution?
( K a = 1.9×10 - 5)
A) 4.42
B) 5.02
C) 9.58
D) 4.72
E) 8.98
( K a = 1.9×10 - 5)
A) 4.42
B) 5.02
C) 9.58
D) 4.72
E) 8.98

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following mixtures would not be suitable for preparing a buffer solution?
A) NH4Cl and NH3
B) HNO3 and NaNO3
C) HF and KF
D) C6H5COOH (Benzoic acid) and C6H5COO - Na+
E) CH3COOH and CH3COO - Na+
A) NH4Cl and NH3
B) HNO3 and NaNO3
C) HF and KF
D) C6H5COOH (Benzoic acid) and C6H5COO - Na+
E) CH3COOH and CH3COO - Na+

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
40
A buffer solution maintains a constant pH level to a certain extent when a strong acid such as HCl is added. Which of the following buffer solutions has the greatest buffering capacity to consume added HCl?
A) 0.5 M HC2H3O2/0.5 M NaC2H3O2
B) 1.0 M HC2H3O2/0.5 M NaC2H3O2
C) 1.0 M HC2H3O2/1.5 M NaC2H3O2
D) 1.5 M HC2H3O2/1.0 M NaC2H3O2
E) 1.5 M HC2H3O2/0.5 M NaC2H3O2
A) 0.5 M HC2H3O2/0.5 M NaC2H3O2
B) 1.0 M HC2H3O2/0.5 M NaC2H3O2
C) 1.0 M HC2H3O2/1.5 M NaC2H3O2
D) 1.5 M HC2H3O2/1.0 M NaC2H3O2
E) 1.5 M HC2H3O2/0.5 M NaC2H3O2

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
41
What is the pH after the addition of 0.20 mole of HCl to 1.00 liter of 1.00 M CH3COONa and 1.00 M CH3COOH ( K a = 1.8×10 - 5) buffer solution?
(Assume no volume change.)
A) 0.70
B) 2.31
C) 4.57
D) 9.28
E) 13.3
(Assume no volume change.)
A) 0.70
B) 2.31
C) 4.57
D) 9.28
E) 13.3

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
42
A buffer was prepared by mixing 0.60 moles of HF and 0.40 moles of NaF in enough water to make 1.00 L of solution. What is the pH of this solution?
K a = 3.5×10 - 4 for HF.
A) 10.72
B) 3.48
C) 3.58
D) 10.42
E) 3.28
K a = 3.5×10 - 4 for HF.
A) 10.72
B) 3.48
C) 3.58
D) 10.42
E) 3.28

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
43
Calculate the amount of ammonium chloride (NH4Cl) that must be added to 1.0 L of 0.10 M NH3 ( K b = 1.8×10 - 5) to prepare a buffer of pH 9.00.
A) 0.10 mole
B) 0.056 mole
C) 0.18 mole
D) 5.6×10 - 5 mole
E) buffer cannot be made
A) 0.10 mole
B) 0.056 mole
C) 0.18 mole
D) 5.6×10 - 5 mole
E) buffer cannot be made

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
44
A 0.10 M solution of hydrazine (N2H4, K b = 1.7×10 - 6) containing an unknown concentration of hydrazine hydrochloride (N2H5Cl) has a pH of 7.50. What is the concentration of N2H5Cl in the solution?
A) 1.26 M
B) 0.54 M
C) 5.8 M
D) 0.10 M
E) 0.72 M
A) 1.26 M
B) 0.54 M
C) 5.8 M
D) 0.10 M
E) 0.72 M

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
45
How many grams of sodium acetate, CH3COONa, is required to mix witH₂ .00 Liters of 0.450 M acetic acid, CH3COOH, in order to prepare a buffer solution with a pH that equals 5.00?
P K a(CH3COOH) = 4.74 and MM(CH3COONa) = 82.03 g/mol
A) 2.00×10 - 2 g CH3COONa
B) 45.6 g CH3COONa
C) 67.2 g CH3COONa
D) 134 g CH3COONa
E) 299 g CH3COONa
P K a(CH3COOH) = 4.74 and MM(CH3COONa) = 82.03 g/mol
A) 2.00×10 - 2 g CH3COONa
B) 45.6 g CH3COONa
C) 67.2 g CH3COONa
D) 134 g CH3COONa
E) 299 g CH3COONa

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
46
A buffer was prepared such that the pH was 5.20. If the solution contains 0.400 moles of acetic acid (CH3COOH) per liter, what is the concentration of the acetate ion (CH3COO - ) in this solution?
The p K a for acetic acid is 4.74.
A) 0.40 M
B) 0.14 M
C) 4.2×10 - 3 M
D) 6.3×10 - 6 M
E) 1.1 M
The p K a for acetic acid is 4.74.
A) 0.40 M
B) 0.14 M
C) 4.2×10 - 3 M
D) 6.3×10 - 6 M
E) 1.1 M

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
47
Exhibit 16-1 Consider the equilibrium reaction below to answer the following question(s). HNO₂ + H₂ O ![<strong>Exhibit 16-1 Consider the equilibrium reaction below to answer the following question(s). HNO₂ + H₂ O H₃O + + NO₂ 1 - K a (HNO₂ ) = 4.5 10 - 4 Refer to Exhibit 16-1. What is the concentration of nitrite ion, NO₂ - , present at equilibrium if 0.100 moles of HCl are added to 0.200 moles of HNO₂ to form one-liter of solution?</strong> A) [NO<sub>2</sub><sup> - </sup>] = 9.00×10<sup> - 4</sup> M B) [NO<sub>2</sub><sup> - </sup>] = 2.12×10<sup> - 3</sup> M C) [NO<sub>2</sub><sup> - </sup>] = 9.49×10<sup> - 3</sup> M D) [NO<sub>2</sub><sup> - </sup>] = 0.100 M E) [NO<sub>2</sub><sup> - </sup>] = 0.200 M](https://storage.examlex.com/TBX8714/11ebff50_c8c3_2262_8da6_116167ac28d4_TBX8714_11.jpg) H₃O + + NO₂ 1 - K a (HNO₂ ) = 4.5 10 - 4
H₃O + + NO₂ 1 - K a (HNO₂ ) = 4.5 10 - 4
Refer to Exhibit 16-1. What is the concentration of nitrite ion, NO₂ - , present at equilibrium if 0.100 moles of HCl are added to 0.200 moles of HNO₂ to form one-liter of solution?
A) [NO2 - ] = 9.00×10 - 4 M
B) [NO2 - ] = 2.12×10 - 3 M
C) [NO2 - ] = 9.49×10 - 3 M
D) [NO2 - ] = 0.100 M
E) [NO2 - ] = 0.200 M
![<strong>Exhibit 16-1 Consider the equilibrium reaction below to answer the following question(s). HNO₂ + H₂ O H₃O + + NO₂ 1 - K a (HNO₂ ) = 4.5 10 - 4 Refer to Exhibit 16-1. What is the concentration of nitrite ion, NO₂ - , present at equilibrium if 0.100 moles of HCl are added to 0.200 moles of HNO₂ to form one-liter of solution?</strong> A) [NO<sub>2</sub><sup> - </sup>] = 9.00×10<sup> - 4</sup> M B) [NO<sub>2</sub><sup> - </sup>] = 2.12×10<sup> - 3</sup> M C) [NO<sub>2</sub><sup> - </sup>] = 9.49×10<sup> - 3</sup> M D) [NO<sub>2</sub><sup> - </sup>] = 0.100 M E) [NO<sub>2</sub><sup> - </sup>] = 0.200 M](https://storage.examlex.com/TBX8714/11ebff50_c8c3_2262_8da6_116167ac28d4_TBX8714_11.jpg) H₃O + + NO₂ 1 - K a (HNO₂ ) = 4.5 10 - 4
H₃O + + NO₂ 1 - K a (HNO₂ ) = 4.5 10 - 4Refer to Exhibit 16-1. What is the concentration of nitrite ion, NO₂ - , present at equilibrium if 0.100 moles of HCl are added to 0.200 moles of HNO₂ to form one-liter of solution?
A) [NO2 - ] = 9.00×10 - 4 M
B) [NO2 - ] = 2.12×10 - 3 M
C) [NO2 - ] = 9.49×10 - 3 M
D) [NO2 - ] = 0.100 M
E) [NO2 - ] = 0.200 M

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
48
What is the pH of a solution that consists of 0.25 M HC2H3O2 and 0.35 M NaC2H3O2?
K a(HC2H3O2) = 1.8×10 - 5
A) 0.60
B) 4.60
C) 4.89
D) 5.50
E) 6.07
K a(HC2H3O2) = 1.8×10 - 5
A) 0.60
B) 4.60
C) 4.89
D) 5.50
E) 6.07

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
49
What is the concentration of ammonium ion, [NH4+], present at equilibrium if 0.100 moles of NaOH are added to 0.200 moles of NH3 to form one-liter of solution?
A) [NH4+] = 9.0×10 - 6 M
B) [NH4+] = 3.6×10 - 5 M
C) [NH4+] = 1.9×10 - 3 M
D) [NH4+] = 6.0×10 - 3 M
E) [NH4+] = 0.10 M
A) [NH4+] = 9.0×10 - 6 M
B) [NH4+] = 3.6×10 - 5 M
C) [NH4+] = 1.9×10 - 3 M
D) [NH4+] = 6.0×10 - 3 M
E) [NH4+] = 0.10 M

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following titration curves listed below best represents a curve for the complete titration of oxalic acid, H2C2O4 (a diprotic acid), with a strong base such as NaOH?
A)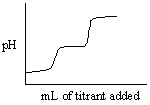
B)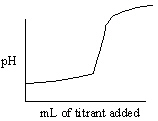
C)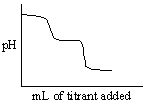
D)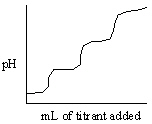
E)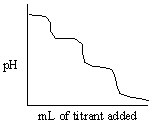
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
51
Consider the ionization of acetic acid as follows:CH ₃COOH + H₂ O  H₃O + + CH₃COO 1 - What is the effect of adding the common ion, acetate, CH₃COO - (source:
H₃O + + CH₃COO 1 - What is the effect of adding the common ion, acetate, CH₃COO - (source:
CH₃COONa), to this equilibrium system?
A) The equilibrium shifts to the left and the pH rises.
B) The equilibrium shifts to the right and the pH rises.
C) The equilibrium shifts to the left and the pH lowers.
D) The equilibrium shifts to the right and the pH lowers.
E) Nothing happens. The pH remains constant and the equilibrium does not shift in either direction.
 H₃O + + CH₃COO 1 - What is the effect of adding the common ion, acetate, CH₃COO - (source:
H₃O + + CH₃COO 1 - What is the effect of adding the common ion, acetate, CH₃COO - (source:CH₃COONa), to this equilibrium system?
A) The equilibrium shifts to the left and the pH rises.
B) The equilibrium shifts to the right and the pH rises.
C) The equilibrium shifts to the left and the pH lowers.
D) The equilibrium shifts to the right and the pH lowers.
E) Nothing happens. The pH remains constant and the equilibrium does not shift in either direction.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
52
What is the pH of a buffer solution that consists of 0.25 M HClO2 and 0.75 M KClO2?
K a(HClO2) = 1.1×10 - 2
A) pH = 1.48
B) pH = 1.96
C) pH = 2.44
D) pH = 3.41
E) pH = 5.61
K a(HClO2) = 1.1×10 - 2
A) pH = 1.48
B) pH = 1.96
C) pH = 2.44
D) pH = 3.41
E) pH = 5.61

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
53
What is the pH after the addition of 0.050 moles of NaOH to 1.00 L of a 0.500 M NH3 / 0.500 M NH4Cl buffer solution?
Assume that there is no volume change on addition of the NaOH. K b for NH3 = 1.8×10 - 5.
A) 4.74
B) 4.66
C) 9.34
D) 9.17
E) 9.53
Assume that there is no volume change on addition of the NaOH. K b for NH3 = 1.8×10 - 5.
A) 4.74
B) 4.66
C) 9.34
D) 9.17
E) 9.53

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
54
A buffer is made by dissolving 0.10 mol of NaF in 1.00 L of 0.20 M HF. What is the pH of this buffer?
K a(HF) = 6.3×10 - 4
A) 1.05
B) 1.58
C) 3.16
D) 3.46
E) 3.70
K a(HF) = 6.3×10 - 4
A) 1.05
B) 1.58
C) 3.16
D) 3.46
E) 3.70

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
55
Consider the ionization of hydrofluoric acid as follows:
HF + H₂ O H₃O + + F 1 - What is the effect of adding the common ion, F - (source:
H₃O + + F 1 - What is the effect of adding the common ion, F - (source:
NaF), to this equilibrium system?
A) The equilibrium shifts to the right and the pH lowers.
B) The equilibrium shifts to the left and the pH lowers.
C) The equilibrium shifts to the right and the pH rises.
D) The equilibrium shifts to the left and the pH rises.
E) Nothing happens. The pH remains constant and the equilibrium does not shift in either direction.
HF + H₂ O
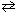 H₃O + + F 1 - What is the effect of adding the common ion, F - (source:
H₃O + + F 1 - What is the effect of adding the common ion, F - (source:NaF), to this equilibrium system?
A) The equilibrium shifts to the right and the pH lowers.
B) The equilibrium shifts to the left and the pH lowers.
C) The equilibrium shifts to the right and the pH rises.
D) The equilibrium shifts to the left and the pH rises.
E) Nothing happens. The pH remains constant and the equilibrium does not shift in either direction.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
56
What is the pH of an aqueous solution that contains 0.183 M sodium formate (NaCHO2) and 0.300 M in formic acid?
K a(HCHO2) = 1.8×10 - 4
A) pH = 3.53
B) pH = 3.96
C) pH = 4.56
D) pH = 5.16
E) pH = 8.13
K a(HCHO2) = 1.8×10 - 4
A) pH = 3.53
B) pH = 3.96
C) pH = 4.56
D) pH = 5.16
E) pH = 8.13

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
57
Exhibit 16-1 Consider the equilibrium reaction below to answer the following question(s). HNO₂ + H₂ O 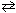 H₃O + + NO₂ 1 - K a (HNO₂ ) = 4.5 10 - 4
H₃O + + NO₂ 1 - K a (HNO₂ ) = 4.5 10 - 4
Refer to Exhibit 16-1. What is the effect of adding NaNO₂ to an aqueous solution of HNO₂ as shown in the equilibrium reaction above?
A) The equilibrium will shift left favoring the reactant side and the pH will drop.
B) The equilibrium will shift right favoring the product side and the pH will drop.
C) There will be no shift in the equilibrium reaction and the pH will remain constant.
D) The equilibrium will shift left favoring the reactant side and the pH will rise.
E) The equilibrium will shift right favoring the product side and the pH will rise.
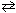 H₃O + + NO₂ 1 - K a (HNO₂ ) = 4.5 10 - 4
H₃O + + NO₂ 1 - K a (HNO₂ ) = 4.5 10 - 4Refer to Exhibit 16-1. What is the effect of adding NaNO₂ to an aqueous solution of HNO₂ as shown in the equilibrium reaction above?
A) The equilibrium will shift left favoring the reactant side and the pH will drop.
B) The equilibrium will shift right favoring the product side and the pH will drop.
C) There will be no shift in the equilibrium reaction and the pH will remain constant.
D) The equilibrium will shift left favoring the reactant side and the pH will rise.
E) The equilibrium will shift right favoring the product side and the pH will rise.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
58
What is the pH of an aqueous solution that contains 0.39 M C6H5COOH and 0.14 M C6H5COONa?
K a(C6H5COOH) = 6.3×10 - 5
A) pH = 0.409
B) pH = 3.76
C) pH = 4.65
D) pH = 6.29
E) pH = 8.65
K a(C6H5COOH) = 6.3×10 - 5
A) pH = 0.409
B) pH = 3.76
C) pH = 4.65
D) pH = 6.29
E) pH = 8.65

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
59
Consider the following equilibrium reaction:
NH₃+ H₂ O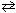 NH ₄+ + OH - K b (NH ₃) = 1.8 10 - 5 Qualitatively, what is the effect of adding NaOH to an aqueous solution of NH₃as shown in the equilibrium reaction above?
NH ₄+ + OH - K b (NH ₃) = 1.8 10 - 5 Qualitatively, what is the effect of adding NaOH to an aqueous solution of NH₃as shown in the equilibrium reaction above?
(Use Le Chatelier s principle.)
A) The equilibrium will shift left favoring the reactant side and the pH will drop.
B) The equilibrium will shift right favoring the product side and the pH will drop.
C) There will be no shift in the equilibrium reaction and the pH will remain constant.
D) The equilibrium will shift left favoring the reactant side and the pH will rise.
E) The equilibrium will shift right favoring the product side and the pH will rise.
NH₃+ H₂ O
 NH ₄+ + OH - K b (NH ₃) = 1.8 10 - 5 Qualitatively, what is the effect of adding NaOH to an aqueous solution of NH₃as shown in the equilibrium reaction above?
NH ₄+ + OH - K b (NH ₃) = 1.8 10 - 5 Qualitatively, what is the effect of adding NaOH to an aqueous solution of NH₃as shown in the equilibrium reaction above?(Use Le Chatelier s principle.)
A) The equilibrium will shift left favoring the reactant side and the pH will drop.
B) The equilibrium will shift right favoring the product side and the pH will drop.
C) There will be no shift in the equilibrium reaction and the pH will remain constant.
D) The equilibrium will shift left favoring the reactant side and the pH will rise.
E) The equilibrium will shift right favoring the product side and the pH will rise.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
60
Calculate the number of moles of sodium acetate (CH3COONa) that must be added to 1.00 L of 0.100 M CH3COOH ( K a = 1.8×10 - 5) to give a buffer with pH5.00.
A) 1.0×10 - 5
B) 0.18
C) 0.057
D) 0.10
E) 0.20
A) 1.0×10 - 5
B) 0.18
C) 0.057
D) 0.10
E) 0.20

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
61
The titration of a weak acid, HA ( K a = 1.0×10 - 4), with NaOH requires 40.0 mL to reach the equivalence point. The pH after 20 mL is
A) Strongly alkaline, approximately 12
B) Moderately alkaline, approximately 10
C) Slightly alkaline, approximately 8
D) Neutral, approximately 7
E) Moderately acidic, approximately 4
A) Strongly alkaline, approximately 12
B) Moderately alkaline, approximately 10
C) Slightly alkaline, approximately 8
D) Neutral, approximately 7
E) Moderately acidic, approximately 4

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following titration curves listed below best represents a curve for the complete titration of the weakly basic dianion, sulfide, S2 - , with a strong acid such as HCl as shown below in the net ionic equation?
S2 - (aq) + 2 HCl (aq)→H2S (g) + 2 Cl -
A)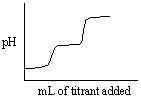
B)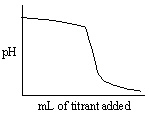
C)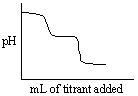
D)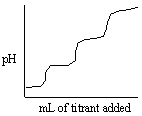
E)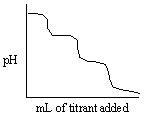
S2 - (aq) + 2 HCl (aq)→H2S (g) + 2 Cl -
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
63
The pH at the equivalence point in the titration of 0.10 M NH3 with 0.10 M HCl is:
A) strongly acidic.
B) slightly acidic.
C) neutral (pH 7.0).
D) slightly basic.
E) strongly basic.
A) strongly acidic.
B) slightly acidic.
C) neutral (pH 7.0).
D) slightly basic.
E) strongly basic.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
64
Exhibit 16-3 Consider the Titration curve below for the titration of a weak acid ,CH ₃COOH , with a strong base , NaOH, to answer the following question(s). CH ₃COOH + NaOH CH ₃COONa + H₂ O ![<strong>Exhibit 16-3 Consider the Titration curve below for the titration of a weak acid ,CH ₃COOH , with a strong base , NaOH, to answer the following question(s). CH ₃COOH + NaOH CH ₃COONa + H₂ O Refer to Exhibit 16-3. What approach listed below would be used to solve for the pH at the equivalence point on this titration curve?</strong> A) The pH at the equivalence point for a weak acid equals 7.0. B) The pH equals the p K <sub>a</sub> of the weak acid at the equivalence point. C) The pH at the equivalence point can be determined by substituting the original molar concentration of the weak acid into the equation: pH = - log[HA]. D) The Henderson-Hasselbalch equation can be used to solve for the pH at the equivalence point. E) The pH at the equivalence point must be solved as a problem of equilibrium involving a basic salt.](https://storage.examlex.com/TBX8714/11ebff50_c8c4_33e8_8da6_7fc3979443a3_TBX8714_00.jpg)
Refer to Exhibit 16-3. What approach listed below would be used to solve for the pH at the equivalence point on this titration curve?
A) The pH at the equivalence point for a weak acid equals 7.0.
B) The pH equals the p K a of the weak acid at the equivalence point.
C) The pH at the equivalence point can be determined by substituting the original molar concentration of the weak acid into the equation: pH = - log[HA].
D) The Henderson-Hasselbalch equation can be used to solve for the pH at the equivalence point.
E) The pH at the equivalence point must be solved as a problem of equilibrium involving a basic salt.
![<strong>Exhibit 16-3 Consider the Titration curve below for the titration of a weak acid ,CH ₃COOH , with a strong base , NaOH, to answer the following question(s). CH ₃COOH + NaOH CH ₃COONa + H₂ O Refer to Exhibit 16-3. What approach listed below would be used to solve for the pH at the equivalence point on this titration curve?</strong> A) The pH at the equivalence point for a weak acid equals 7.0. B) The pH equals the p K <sub>a</sub> of the weak acid at the equivalence point. C) The pH at the equivalence point can be determined by substituting the original molar concentration of the weak acid into the equation: pH = - log[HA]. D) The Henderson-Hasselbalch equation can be used to solve for the pH at the equivalence point. E) The pH at the equivalence point must be solved as a problem of equilibrium involving a basic salt.](https://storage.examlex.com/TBX8714/11ebff50_c8c4_33e8_8da6_7fc3979443a3_TBX8714_00.jpg)
Refer to Exhibit 16-3. What approach listed below would be used to solve for the pH at the equivalence point on this titration curve?
A) The pH at the equivalence point for a weak acid equals 7.0.
B) The pH equals the p K a of the weak acid at the equivalence point.
C) The pH at the equivalence point can be determined by substituting the original molar concentration of the weak acid into the equation: pH = - log[HA].
D) The Henderson-Hasselbalch equation can be used to solve for the pH at the equivalence point.
E) The pH at the equivalence point must be solved as a problem of equilibrium involving a basic salt.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following titrations would produce a pH equal to 7.0 at the equivalence point?
I. Titrating NH3 with standard HCl (delivered from the burette)
II. Titrating HCl with standard NaOH (delivered from the burette)
III. Titrating CH3COOH with standard NaOH (delivered from the burette)
A) I only
B) II only
C) III only
D) II and III
E) All of these
I. Titrating NH3 with standard HCl (delivered from the burette)
II. Titrating HCl with standard NaOH (delivered from the burette)
III. Titrating CH3COOH with standard NaOH (delivered from the burette)
A) I only
B) II only
C) III only
D) II and III
E) All of these

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
66
Consider titrating a triprotic acid with standard NaOH as shown in the titration curve below. 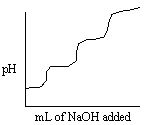 What is the relationship between the first equivalence point and the third equivalence point in this titration curve?
What is the relationship between the first equivalence point and the third equivalence point in this titration curve?
A) The pH of the first equivalence point is three times larger than the pH found at the third equivalence point.
B) The pH of the first equivalence point is three times smaller than the pH found at the third equivalence point.
C) The volume of titrant required to reach the third equivalence point is three times as large as the volume of titrant required to reach the first equivalence point.
D) The volume of titrant required to reach the first equivalence point is three times as large as the volume of titrant required to reach the third equivalence point.
E) There are no relationships between the first and third equivalence points.
 What is the relationship between the first equivalence point and the third equivalence point in this titration curve?
What is the relationship between the first equivalence point and the third equivalence point in this titration curve?A) The pH of the first equivalence point is three times larger than the pH found at the third equivalence point.
B) The pH of the first equivalence point is three times smaller than the pH found at the third equivalence point.
C) The volume of titrant required to reach the third equivalence point is three times as large as the volume of titrant required to reach the first equivalence point.
D) The volume of titrant required to reach the first equivalence point is three times as large as the volume of titrant required to reach the third equivalence point.
E) There are no relationships between the first and third equivalence points.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
67
The titration of a weak acid, HA ( K a = 1.0×10 - 4), with NaOH requires 40.0 mL to reach the equivalence point. In order to calculate the pH of the system after 20 mL of NaOH is added, the best strategy is to recognize that the system is
A) a strong acid
B) a weak acid
C) a buffer
D) neutral
E) a weak base
A) a strong acid
B) a weak acid
C) a buffer
D) neutral
E) a weak base

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
68
In the titration of 25.0 mL of 0.100 M acetic acid ( K a = 1.8×10 - 5) with 0.100 M NaOH, the pH after adding 12.5 mL of titrant is:
A) 12.52
B) 3.50
C) 7.00
D) 4.74
E) 2.38
A) 12.52
B) 3.50
C) 7.00
D) 4.74
E) 2.38

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
69
Consider the titration of a weak acid with a strong base . Which statement below is true regarding the equivalence point in this titration?
A) It occurs at a pH = 7.0 because the acid has been neutralized.
B) It occurs at a pH less than 7.0 because an acidic salt is formed.
C) It occurs at a pH less than 7.0 because a basic salt is formed.
D) It occurs at a pH greater than 7.0 because an acidic salt is formed.
E) It occurs at a pH greater than 7.0 because a basic salt is formed.
A) It occurs at a pH = 7.0 because the acid has been neutralized.
B) It occurs at a pH less than 7.0 because an acidic salt is formed.
C) It occurs at a pH less than 7.0 because a basic salt is formed.
D) It occurs at a pH greater than 7.0 because an acidic salt is formed.
E) It occurs at a pH greater than 7.0 because a basic salt is formed.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
70
A 20.0 mL sample of lactic acid (monoprotic with K a = 1.37×10 - 4) requires 30.0 mL of 0.200 M NaOH for titration to the equivalence point. What is the concentration of the lactic acid solution?
A) 0.120 M
B) 0.200 M
C) 0.300 M
D) 6.00×10 - 3 M
E) cannot be found without the molar mass of lactic acid.
A) 0.120 M
B) 0.200 M
C) 0.300 M
D) 6.00×10 - 3 M
E) cannot be found without the molar mass of lactic acid.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following titration curves listed below best represents a curve for the complete titration of citric acid, H3C6H5O7, a triprotic acid with a strong base such as NaOH?
A)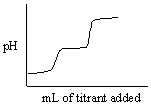
B)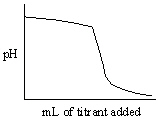
C)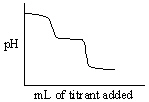
D)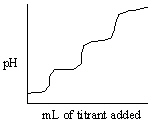
E)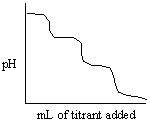
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
72
The titration of a weak base ( K b = 1.0×10 - 4) with HCl requires 40.0 mL to reach the equivalence point. In order to calculate the pH of the system after 20 mL of HCl is added, the best strategy is to recognize that the system is
A) a strong base
B) a weak base
C) a buffer
D) neutral
E) a strong acid
A) a strong base
B) a weak base
C) a buffer
D) neutral
E) a strong acid

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
73
A sample of 0.100 mole of acetic acid ( K a = 1.8×10 - 5) in 50.0 mL of solution is titrated with standard 0.100 M NaOH. What is the pH in the titration flask after addition of 25.0 mL of the base?
A) 2.88
B) 3.18
C) 2.60
D) 4.74
E) 11.40
A) 2.88
B) 3.18
C) 2.60
D) 4.74
E) 11.40

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
74
Exhibit 16-3 Consider the Titration curve below for the titration of a weak acid ,CH ₃COOH , with a strong base , NaOH, to answer the following question(s). CH ₃COOH + NaOH CH ₃COONa + H₂ O 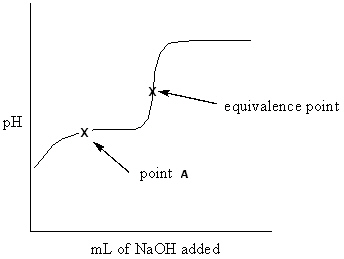
Refer to Exhibit 16-3. What approach listed below would be used to determine the pH at point A of the solution?
A) This is a problem of solving for the pH of an aqueous weak acid solution.
B) This is a problem of solving for the pH of an aqueous weak base solution.
C) This is a problem of solving for the pH of an aqueous buffer solution.
D) This is a problem of solving for the pH of an aqueous strong base solution.
E) This is a problem of solving for the pH of an aqueous salt solution.

Refer to Exhibit 16-3. What approach listed below would be used to determine the pH at point A of the solution?
A) This is a problem of solving for the pH of an aqueous weak acid solution.
B) This is a problem of solving for the pH of an aqueous weak base solution.
C) This is a problem of solving for the pH of an aqueous buffer solution.
D) This is a problem of solving for the pH of an aqueous strong base solution.
E) This is a problem of solving for the pH of an aqueous salt solution.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
75
Exhibit 16-2 Consider titrating CH3COOH with standard NaOH (delivered from the burette) to answer the following question(s).
Refer to Exhibit 16-2. How would the pH be determined for the aqueous solution in the titration above at the equivalence point ?
A) The pH would equal 7.0 at the equivalence point.
B) The pH is determined by the Henderson-Hasselbalch equation.
C) The pH is determined by taking - log[CH3COOH]original.
D) The pH is determined by solving for the pH of a basic salt.
E) The pH is determined by solving for the pH of an acidic salt.
Refer to Exhibit 16-2. How would the pH be determined for the aqueous solution in the titration above at the equivalence point ?
A) The pH would equal 7.0 at the equivalence point.
B) The pH is determined by the Henderson-Hasselbalch equation.
C) The pH is determined by taking - log[CH3COOH]original.
D) The pH is determined by solving for the pH of a basic salt.
E) The pH is determined by solving for the pH of an acidic salt.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
76
What is the pH of solution formed by adding 25.0 mL of 0.100 M HCl to 25.0 mL of 0.100 M pyridine (C5H5N, K b = 1.8×10 - 9)?
A) 2.38
B) 2.52
C) 2.98
D) 3.28
E) 8.74
A) 2.38
B) 2.52
C) 2.98
D) 3.28
E) 8.74

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
77
What is the pH of the following solution?
20)0 mL of 0.50 M acetic acid ( K a = 1.8×10 - 5) is added tO5 .00 mL of 0.50 M NaOH.
A) 4.27
B) 4.74
C) 5.22
D) 5.40
E) 7.00
20)0 mL of 0.50 M acetic acid ( K a = 1.8×10 - 5) is added tO5 .00 mL of 0.50 M NaOH.
A) 4.27
B) 4.74
C) 5.22
D) 5.40
E) 7.00

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
78
The sharpest inflection point is observed in the titration of
A) 0.1 M HCl with 0.10 M NaOH
B) 0.01 M HCl with 0.10 M NaOH
C) 0.1 M KOH with 0.10 M NaOH
D) 0.1 M HF ( Ka = 3.5×10 - 4) with 0.10 M NaOH
E) 0.10 M NH3 with 0.10 M NaOH
A) 0.1 M HCl with 0.10 M NaOH
B) 0.01 M HCl with 0.10 M NaOH
C) 0.1 M KOH with 0.10 M NaOH
D) 0.1 M HF ( Ka = 3.5×10 - 4) with 0.10 M NaOH
E) 0.10 M NH3 with 0.10 M NaOH

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
79
Which experiment listed below would provide a titration curve that resembles the titration shown below?
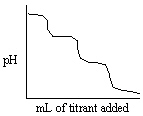
A) Titrating NH3 with standard NaOH (delivered from the burette).
B) Titrating NH3 with standard HCl (delivered from the burette).
C) Titrating H3PO4 with standard NaOH (delivered from the burette).
D) Titrating Na3PO4 with standard HCl (delivered from the burette).
E) Titrating Na3PO4 with standard NaOH (delivered from the burette).

A) Titrating NH3 with standard NaOH (delivered from the burette).
B) Titrating NH3 with standard HCl (delivered from the burette).
C) Titrating H3PO4 with standard NaOH (delivered from the burette).
D) Titrating Na3PO4 with standard HCl (delivered from the burette).
E) Titrating Na3PO4 with standard NaOH (delivered from the burette).

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
80
Exhibit 16-2 Consider titrating CH3COOH with standard NaOH (delivered from the burette) to answer the following question(s).
Refer to Exhibit 16-2. What compound(s) is(are) present in the region after the titration has begun but before the equivalence point?
I. CH3COOH
II. NaOH
III. CH3COO - Na+
A) I only
B) I and II
C) I and III
D) II and III
E) All of these are present.
Refer to Exhibit 16-2. What compound(s) is(are) present in the region after the titration has begun but before the equivalence point?
I. CH3COOH
II. NaOH
III. CH3COO - Na+
A) I only
B) I and II
C) I and III
D) II and III
E) All of these are present.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck


