Deck 9: Hydrocarbons: an Introduction to Organic Molecules
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/74
Play
Full screen (f)
Deck 9: Hydrocarbons: an Introduction to Organic Molecules
1
The following compound is classified as an alkane. 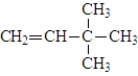
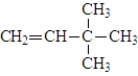
False
2
One of the reasons that carbon forms such a large number of compounds is due its ability to form long chains and rings.
True
3
Examine the structure shown below. 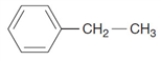 The compound is a hydrocarbon and an aromatic compound
The compound is a hydrocarbon and an aromatic compound
 The compound is a hydrocarbon and an aromatic compound
The compound is a hydrocarbon and an aromatic compoundTrue
4
The compound below is unlikely to exist. 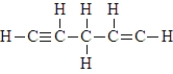
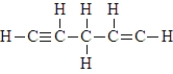

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
5
The following compound contains four tetrahedrally arranged atoms. 


Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
6
The line structure for 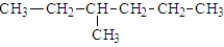 is shown below.
is shown below. 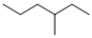
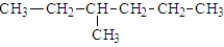 is shown below.
is shown below. 

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
7
The structure for 2,2,3,3-tetramethylbutane is: 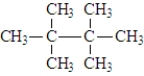
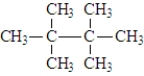

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
8
How many hydrogen atoms would be needed to complete the following structure? 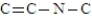
A) 6
B) 7
C) 9
D) 10
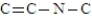
A) 6
B) 7
C) 9
D) 10

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
9
The following structure illustrates a trans isomer. 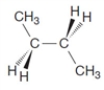


Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
10
The two compounds shown below have the same molecular formula. CH3-CH2-O-CH2-CH2-CH3 CH3-CH2-CH2-CH2-CH2-OH

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
11
The following two substances would have the same physical properties. 


Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
12
The following represents trans-2,3-dimethyl-2-pentene. 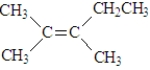


Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
13
In propyne, the arrangement of bonds around all of the carbon atoms is shown below. 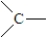


Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
14
The following compound would be correctly classified as an alkyne. 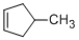


Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
15
The compound shown below is an isomer of 2,2-dimethylbutane. 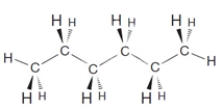


Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
16
The two compounds shown below would be expected to exhibit similiar properties. CH3-CH2-O-CH2-CH2-CH3 CH3-CH2-CH2-CH2-CH2-OH

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
17
Carbon atoms involved in double bonds exhibit a trigonal planar arrangement.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
18
The following molecule contains two functional groups, a haloalkane group and an alkyne. CH3-CHBr-CH=CH2

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
19
In the compound shown below, which atoms, if any, do not form the correct number of covalent bonds? 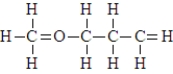
A) H
B) O
C) C
D) H and C
E) C and O
F) H, C and O
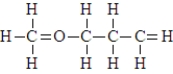
A) H
B) O
C) C
D) H and C
E) C and O
F) H, C and O

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
20
The correct IUPAC name of the following compound is methylcyclohexane. 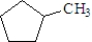


Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
21
The IUPAC name of the following contains an ethyl group located at position 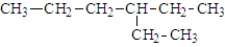
A) 1.
B) 2.
C) 3.
D) 4.
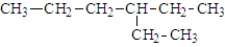
A) 1.
B) 2.
C) 3.
D) 4.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
22
Consider the structure of the compound shown below. 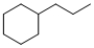 This compound is correctly characterized as:
This compound is correctly characterized as:
A) branched alkane
B) saturated
C) aromatic
D) all of the above
 This compound is correctly characterized as:
This compound is correctly characterized as:A) branched alkane
B) saturated
C) aromatic
D) all of the above

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
23
What is the IUPAC name for compound represented below? 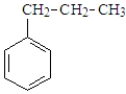
A) isopropylbenzene
B) propylcyclohexane
C) propylbenzene
D) propylcyclohexene

A) isopropylbenzene
B) propylcyclohexane
C) propylbenzene
D) propylcyclohexene

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following is a correct structure for methylcyclopentane?
A)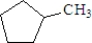
B)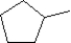
C)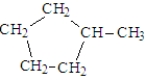
D) All are correct representations.
A)

B)

C)

D) All are correct representations.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following is(are) constitutional isomer(s) of hexane?
A)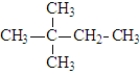
B)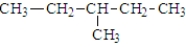
C)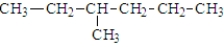
D) both a and b
A)
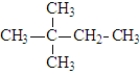
B)
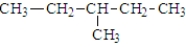
C)
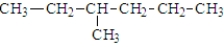
D) both a and b

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
26
Of the compounds listed here, which one is not a constitutional isomer of the other three?
A) 2,3-dimethylpentane
B) 3-methylhexane
C) 3-ethylpentane
D) 2,2-dimethylbutane
A) 2,3-dimethylpentane
B) 3-methylhexane
C) 3-ethylpentane
D) 2,2-dimethylbutane

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
27
A student named a particular compound 2-ethyl-3-methyl-2-butene. Assuming that the student's choice actually corresponded to the correct distribution of the double bond and the substituents, what is the correct IUPAC name for this compound?
A) 2-ethyl-3-methyl-2-butene
B) 3,4-dimethyl-3-pentene
C) 2,3-dimethyl-2-pentene
D) 2,3-dimethyl-1-pentene
A) 2-ethyl-3-methyl-2-butene
B) 3,4-dimethyl-3-pentene
C) 2,3-dimethyl-2-pentene
D) 2,3-dimethyl-1-pentene

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
28
Given the structure below, what is the IUPAC name for the compound? 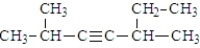
A) 2,5-dimethyl-3-heptyne
B) 5-ethyl-2-methyl-3-hexyne
C) 3,7-dimethyl-4-heptyne
D) 1,1,5-trimethyl-2-hexyne
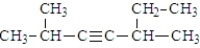
A) 2,5-dimethyl-3-heptyne
B) 5-ethyl-2-methyl-3-hexyne
C) 3,7-dimethyl-4-heptyne
D) 1,1,5-trimethyl-2-hexyne

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following line structures is 4-isopropyloctane?
A)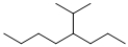
B)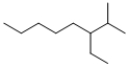
C)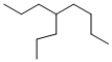
D)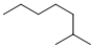
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following line structures represents butane?
A)
B)
C)
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
31
Consider the compounds given in the choices. Which would have the lowest melting point?
A)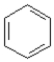
B)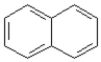
C)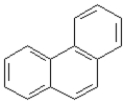
D) All have about the same melting point.
A)

B)

C)

D) All have about the same melting point.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
32
Give the IUPAC name for the following 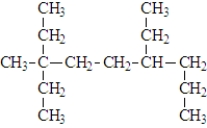
A) 3,6-diethyl 3-methylnonane
B) 2,2,5,6-tetraethylhexane
C) 2,2,5-triethyloctane
D) none are correct
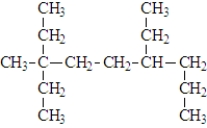
A) 3,6-diethyl 3-methylnonane
B) 2,2,5,6-tetraethylhexane
C) 2,2,5-triethyloctane
D) none are correct

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following hydrocarbons is most likely to be a gas at room temperature?
A) butene
B) 1-hexene
C) 2-octene
D) 4-decene
A) butene
B) 1-hexene
C) 2-octene
D) 4-decene

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
34
What is the IUPAC name for the following alkane? 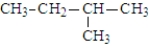
A) 2-ethylpropane
B) methylbutane
C) pentane
D) 3,3-dimethylpropane
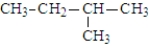
A) 2-ethylpropane
B) methylbutane
C) pentane
D) 3,3-dimethylpropane

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following is could not be used to represent the same compound?
A)
B) CH3-CH2-CH2-CH2-CH3
C) C5H12
D) All could represent the same compound.
A)

B) CH3-CH2-CH2-CH2-CH3
C) C5H12
D) All could represent the same compound.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following would show the most similar chemical behavior to 1-butanol, which is shown below? CH3CH2CH2CH2-OH
A)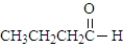
B)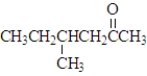
C)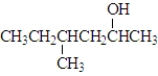
D)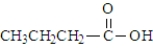
A)
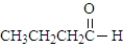
B)
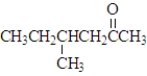
C)
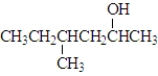
D)
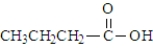

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
37
The following compound would be classified as an: 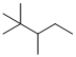
A) alkane
B) alkene
C) alkyne
D) aromatic

A) alkane
B) alkene
C) alkyne
D) aromatic

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following have cis and trans isomeric forms?
A) heptane
B) 2-heptyne
C) 1-heptene
D) 2-heptene
E) Both c and d have cis and trans isomeric forms.
F) All have cis and trans isomeric forms.
A) heptane
B) 2-heptyne
C) 1-heptene
D) 2-heptene
E) Both c and d have cis and trans isomeric forms.
F) All have cis and trans isomeric forms.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
39
What is line bond formula for the following full structural formula? 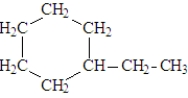
A)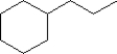
B)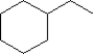
C)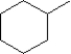
D)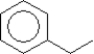

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following is a correct IUPAC name?
A) 1-methylhexane
B) 2-ethylhexane
C) 3-propylhexane
D) None, they are all incorrect.
A) 1-methylhexane
B) 2-ethylhexane
C) 3-propylhexane
D) None, they are all incorrect.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following is probably soluble in water?
A) ethylbenzene
B) xylene
C) 2,2-dimethylbenzene
D) All are insoluble in water.
A) ethylbenzene
B) xylene
C) 2,2-dimethylbenzene
D) All are insoluble in water.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
42
Consider the formula given below. C4H8 This following represents the molecular formula of an
A) alkyne
B) alkene
C) alkane
D) none of these
A) alkyne
B) alkene
C) alkane
D) none of these

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
43
Examine the following structures. 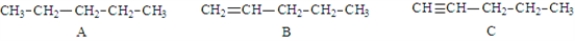 Fill in the blanks with the appropriate letter (A, B, C, ....).
Fill in the blanks with the appropriate letter (A, B, C, ....).
Structure ______________________is most likely to be least reactive.
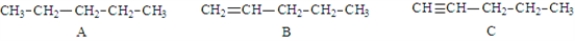 Fill in the blanks with the appropriate letter (A, B, C, ....).
Fill in the blanks with the appropriate letter (A, B, C, ....).Structure ______________________is most likely to be least reactive.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
44
Examine the following structures. 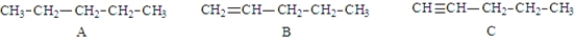 Fill in the blanks with the appropriate letter (A, B, C, ....).
Fill in the blanks with the appropriate letter (A, B, C, ....).
Structure C is classified as a(n) _________________________.
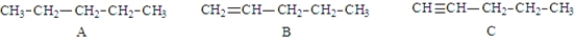 Fill in the blanks with the appropriate letter (A, B, C, ....).
Fill in the blanks with the appropriate letter (A, B, C, ....).Structure C is classified as a(n) _________________________.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
45
Consider the following structures. 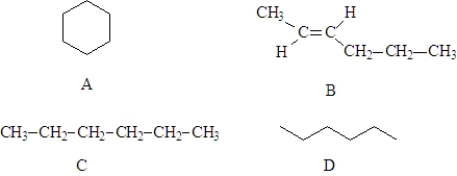 Fill in the blanks with the appropriate letters (A, B, C, or D).
Fill in the blanks with the appropriate letters (A, B, C, or D).
Structure ______________________ is a stereoisomer of cis-2-hexene.
 Fill in the blanks with the appropriate letters (A, B, C, or D).
Fill in the blanks with the appropriate letters (A, B, C, or D).Structure ______________________ is a stereoisomer of cis-2-hexene.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
46
Consider the following structure. 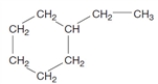 What is the relationship to the name given below? 2,3-dimethylhexane
What is the relationship to the name given below? 2,3-dimethylhexane
A) constitutional isomers
B) cis-trans isomers
C) same compound
D) cycloalkane
E) diffferent compound
 What is the relationship to the name given below? 2,3-dimethylhexane
What is the relationship to the name given below? 2,3-dimethylhexaneA) constitutional isomers
B) cis-trans isomers
C) same compound
D) cycloalkane
E) diffferent compound

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
47
Examine the following structures. 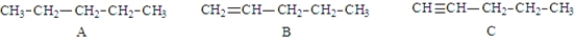 Fill in the blanks with the appropriate letter (A, B, C, ....).
Fill in the blanks with the appropriate letter (A, B, C, ....).
Structure D is classified as a(n) _________________________.
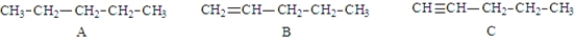 Fill in the blanks with the appropriate letter (A, B, C, ....).
Fill in the blanks with the appropriate letter (A, B, C, ....).Structure D is classified as a(n) _________________________.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
48
Consider the following molecule of the birth control drug known as RU 486. 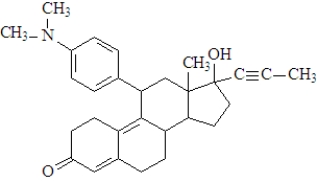 Fill in the blanks with the appropriate term from the list below.
Fill in the blanks with the appropriate term from the list below.
alkane
cycloalkane
alkene
cycloalkene
alkyne
aromatic
haloalkane
The functional group enclosed in the oval is a(n)_________________________.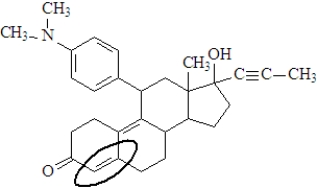
 Fill in the blanks with the appropriate term from the list below.
Fill in the blanks with the appropriate term from the list below. alkane
cycloalkane
alkene
cycloalkene
alkyne
aromatic
haloalkane
The functional group enclosed in the oval is a(n)_________________________.


Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
49
Examine the following structures. 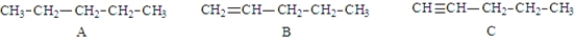 Fill in the blanks with the appropriate letter (A, B, C, ....).
Fill in the blanks with the appropriate letter (A, B, C, ....).
Structure B is classified as a(n) _________________________.
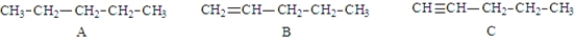 Fill in the blanks with the appropriate letter (A, B, C, ....).
Fill in the blanks with the appropriate letter (A, B, C, ....).Structure B is classified as a(n) _________________________.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
50
Examine the following structures. 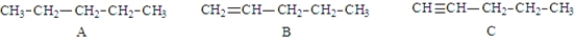 Fill in the blanks with the appropriate letter (A, B, C, ....).
Fill in the blanks with the appropriate letter (A, B, C, ....).
Structure A is classified as a(n) _________________________.
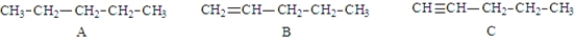 Fill in the blanks with the appropriate letter (A, B, C, ....).
Fill in the blanks with the appropriate letter (A, B, C, ....).Structure A is classified as a(n) _________________________.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
51
Consider the following molecule of the birth control drug known as RU 486.  Fill in the blanks with the appropriate term from the list below.
Fill in the blanks with the appropriate term from the list below.
alkane
cycloalkane
alkene
cycloalkene
alkyne
aromatic
haloalkane
The functional group enclosed in the square is a(n)_________________________.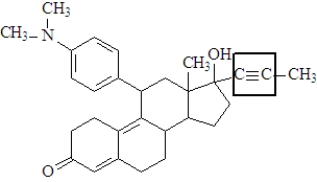
 Fill in the blanks with the appropriate term from the list below.
Fill in the blanks with the appropriate term from the list below. alkane
cycloalkane
alkene
cycloalkene
alkyne
aromatic
haloalkane
The functional group enclosed in the square is a(n)_________________________.


Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
52
Consider the following structure. 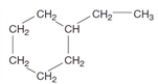 What is the relationship to the structure given below? CH3-CH2-CH2-CH=CH-CH2-CH2-CH3
What is the relationship to the structure given below? CH3-CH2-CH2-CH=CH-CH2-CH2-CH3
A) constitutional isomers.
B) cis-trans isomers.
C) same compound.
D) cycloalkane.
E) different compound.
 What is the relationship to the structure given below? CH3-CH2-CH2-CH=CH-CH2-CH2-CH3
What is the relationship to the structure given below? CH3-CH2-CH2-CH=CH-CH2-CH2-CH3A) constitutional isomers.
B) cis-trans isomers.
C) same compound.
D) cycloalkane.
E) different compound.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
53
What is the name of a branch on the principal carbon chain that contains three carbon atoms?
A) pentyl
B) propyl
C) ethyl
D) methyl
A) pentyl
B) propyl
C) ethyl
D) methyl

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
54
Consider the following structures.  Fill in the blanks with the appropriate letters (A, B, C, or D).
Fill in the blanks with the appropriate letters (A, B, C, or D).
Structure A is a constitutional isomer of Structure _____________________.
 Fill in the blanks with the appropriate letters (A, B, C, or D).
Fill in the blanks with the appropriate letters (A, B, C, or D).Structure A is a constitutional isomer of Structure _____________________.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
55
What must be the name for the missing hydrocarbon in the following combustion reaction? _______ + 3 O2 → 2 CO2 + 2 H2O
A) ethane
B) ethene
C) ethyne
D) More than one hydrocarbon is possible.
A) ethane
B) ethene
C) ethyne
D) More than one hydrocarbon is possible.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
56
Consider the following molecule of the birth control drug known as RU 486.  Fill in the blanks with the appropriate term from the list below.
Fill in the blanks with the appropriate term from the list below.
alkane
cycloalkane
alkene
cycloalkene
alkyne
aromatic
haloalkane
The functional group enclosed in the triangle is a(n)_____________________.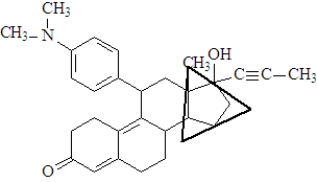
 Fill in the blanks with the appropriate term from the list below.
Fill in the blanks with the appropriate term from the list below. alkane
cycloalkane
alkene
cycloalkene
alkyne
aromatic
haloalkane
The functional group enclosed in the triangle is a(n)_____________________.


Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
57
Consider the following molecule of the birth control drug known as RU 486.  Fill in the blanks with the appropriate term from the list below.
Fill in the blanks with the appropriate term from the list below.
alkane
cycloalkane
alkene
cycloalkene
alkyne
aromatic
haloalkane
The functional group enclosed in the circle is a(n)_____________________.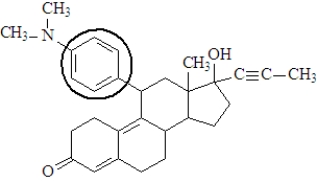
 Fill in the blanks with the appropriate term from the list below.
Fill in the blanks with the appropriate term from the list below. alkane
cycloalkane
alkene
cycloalkene
alkyne
aromatic
haloalkane
The functional group enclosed in the circle is a(n)_____________________.


Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following is the appropriate ending for the name of an alkane?
A) ane
B) ene
C) benzene
D) yne
A) ane
B) ene
C) benzene
D) yne

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
59
Consider the following structure. 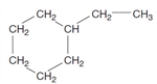 This substance would be classified as a(n)
This substance would be classified as a(n)
A) alkane.
B) alkene.
C) alkyne.
D) aromatic hydrocarbon.
E) cycloalkane.
 This substance would be classified as a(n)
This substance would be classified as a(n)A) alkane.
B) alkene.
C) alkyne.
D) aromatic hydrocarbon.
E) cycloalkane.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
60
Consider the following structures.  Fill in the blanks with the appropriate letters (A, B, C, or D).
Fill in the blanks with the appropriate letters (A, B, C, or D).
Structure C is identical to Structure _____________________.
 Fill in the blanks with the appropriate letters (A, B, C, or D).
Fill in the blanks with the appropriate letters (A, B, C, or D).Structure C is identical to Structure _____________________.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
61
Using molecular formula, write the balance equation for the complete combustion of benzene.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
62
Examine the following structure. 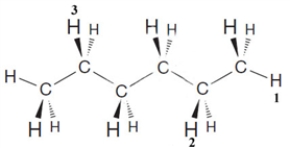 Complete the following statements about this structure.
Complete the following statements about this structure.
If all three numbered hydrogen atoms were replaced by a methyl group, the IUPAC name of the new compound would be ____________.
 Complete the following statements about this structure.
Complete the following statements about this structure.If all three numbered hydrogen atoms were replaced by a methyl group, the IUPAC name of the new compound would be ____________.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
63
Examine the following structures. 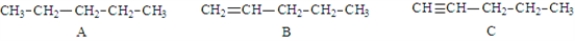 Fill in the blanks with the appropriate letter (A, B, C, ....).
Fill in the blanks with the appropriate letter (A, B, C, ....).
Structure ______________________ would have chemical and physical properties closest to that of 2-hexene.
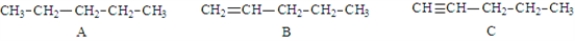 Fill in the blanks with the appropriate letter (A, B, C, ....).
Fill in the blanks with the appropriate letter (A, B, C, ....).Structure ______________________ would have chemical and physical properties closest to that of 2-hexene.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
64
Draw the condensed structural formula of 3-ethyl-3-methylhexane.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
65
Examine the following structure.  Complete the following statements about this structure.
Complete the following statements about this structure.
The IUPAC name for this substance is____________.
 Complete the following statements about this structure.
Complete the following statements about this structure.The IUPAC name for this substance is____________.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
66
Examine the following structures. 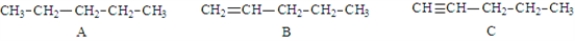 Fill in the blanks with the appropriate letter (A, B, C, ....).
Fill in the blanks with the appropriate letter (A, B, C, ....).
Structure ____________________ will have the lowest melting point.
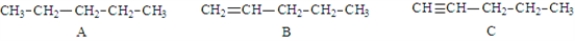 Fill in the blanks with the appropriate letter (A, B, C, ....).
Fill in the blanks with the appropriate letter (A, B, C, ....).Structure ____________________ will have the lowest melting point.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
67
What is the condensed structural formula for the following substance? 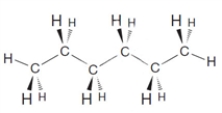


Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
68
Examine the following structure.  Complete the following statements about this structure.
Complete the following statements about this structure.
If the hydrogen atom numbered "1" replaced by a methyl group, the IUPAC name of the new compound would be ____________.
 Complete the following statements about this structure.
Complete the following statements about this structure.If the hydrogen atom numbered "1" replaced by a methyl group, the IUPAC name of the new compound would be ____________.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
69
What is the name of the following compound? 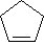


Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
70
Examine the following structure.  Complete the following statements about this structure.
Complete the following statements about this structure.
If the hydrogen atom numbered "2" replaced by a methyl group, the IUPAC name of the new compound would be ____________.
 Complete the following statements about this structure.
Complete the following statements about this structure.If the hydrogen atom numbered "2" replaced by a methyl group, the IUPAC name of the new compound would be ____________.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
71
Examine the following structures. 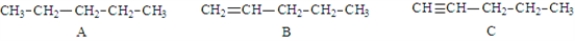 Fill in the blanks with the appropriate letter (A, B, C, ....).
Fill in the blanks with the appropriate letter (A, B, C, ....).
The balanced equation for the combustion of structure _______________________ would required the largest coefficient for O2.
 Fill in the blanks with the appropriate letter (A, B, C, ....).
Fill in the blanks with the appropriate letter (A, B, C, ....).The balanced equation for the combustion of structure _______________________ would required the largest coefficient for O2.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
72
What is the molecular formula for the following substance? 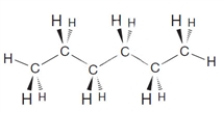


Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
73
Examine the following structure.  Complete the following statements about this structure.
Complete the following statements about this structure.
The arrangement of atoms about each of the carbon atoms is a ____________.
 Complete the following statements about this structure.
Complete the following statements about this structure.The arrangement of atoms about each of the carbon atoms is a ____________.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
74
During the complete combustion of benzene, if 9.55 g of benzene were consumed what mass of oxygen in grams would be needed?

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck


