Deck 7: Aqueous Solutions: Part Ii
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/122
Play
Full screen (f)
Deck 7: Aqueous Solutions: Part Ii
1
What is Pseudomonas aeruginosa?
A)A bacteria found in air and warm water
B)An antibiotic-resistant bacteria
C)A gut bacteria in humans and other mammals
D)A benign bacteria
A)A bacteria found in air and warm water
B)An antibiotic-resistant bacteria
C)A gut bacteria in humans and other mammals
D)A benign bacteria
B
2
What helps produce drug-resistant bacteria?
A)Exposure to warm growth conditions in hospitals
B)Lack of antibiotics
C)Exposure to antibiotics
D)Exposure to antibiotics that do not kill off all infectious bacteria during an exposure
A)Exposure to warm growth conditions in hospitals
B)Lack of antibiotics
C)Exposure to antibiotics
D)Exposure to antibiotics that do not kill off all infectious bacteria during an exposure
D
3
What is another, older name for 1,2-ethanediol?
A)Glyocol
B)Ethylene
C)Ethanol
D)Ethylene glycol
A)Glyocol
B)Ethylene
C)Ethanol
D)Ethylene glycol
D
4
What percentage of the approximately 25 million known compounds contain carbon?
A)Over 25%
B)50%
C)Over 90%
D)100%
A)Over 25%
B)50%
C)Over 90%
D)100%

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
5
What is the name of one compound with the molecular formula C4H10?
A)Butane
B)Propane
C)Ethane
D)Methane
A)Butane
B)Propane
C)Ethane
D)Methane

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
6
What is an organic compound?
A)A compound composed primarily of carbon and oxygen atoms
B)A compound composed primarily of carbon and nitrogen atoms
C)A compound composed primarily of hydrogen and oxygen atoms
D)A compound composed primarily of carbon and hydrogen atoms
A)A compound composed primarily of carbon and oxygen atoms
B)A compound composed primarily of carbon and nitrogen atoms
C)A compound composed primarily of hydrogen and oxygen atoms
D)A compound composed primarily of carbon and hydrogen atoms

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
7
A covalent bond is an example of what kind of force?
A)An intermolecular force
B)An interatomic force
C)An intramolecular force
D)An intra-atomic force
A)An intermolecular force
B)An interatomic force
C)An intramolecular force
D)An intra-atomic force

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
8
Intermolecular forces are able to affect what physical properties of matter?
A)Condensation point
B)Freezing point
C)Both of the above
D)Neither of the above
A)Condensation point
B)Freezing point
C)Both of the above
D)Neither of the above

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
9
Why are alkanes commonly used as solvents?
A)They are fairly non-reactive.
B)They are light sensitive.
C)They are non-toxic.
D)They are easily disposed of.
A)They are fairly non-reactive.
B)They are light sensitive.
C)They are non-toxic.
D)They are easily disposed of.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
10
What is the correct Lewis structure for pentane?
A)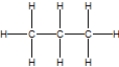
B)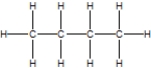
C)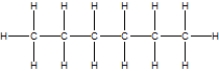
D)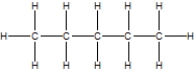
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is the correct Lewis structure for ethane?
A)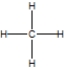
B)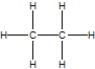
C)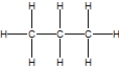
D)None of the above.
A)

B)

C)

D)None of the above.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
12
Of the following, which best represents octane?
A)
B)
C)
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
13
The correct condensed structural formula for propane is CH3CH2CH2CH3.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
14
How can a dipole in a molecule be induced?
A)Through interaction with a temporary dipole in a neighboring molecule
B)Through cooling the system
C)By adjusting the size of the electron cloud
D)A dipole cannot be induced.
A)Through interaction with a temporary dipole in a neighboring molecule
B)Through cooling the system
C)By adjusting the size of the electron cloud
D)A dipole cannot be induced.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
15
Which is the strongest intermolecular force?
A)Dispersion forces
B)Van der Waals forces
C)London forces
D)None of the above; they are all the same.
A)Dispersion forces
B)Van der Waals forces
C)London forces
D)None of the above; they are all the same.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
16
Dipole-dipole forces are found in what type of matter?
A)In compounds that are nonpolar and have a permanent dipole
B)In compounds that are polar and have a permanent dipole
C)In compounds that are polar and do not have a permanent dipole
D)In compounds that are nonpolar and that have no permanent dipole
A)In compounds that are nonpolar and have a permanent dipole
B)In compounds that are polar and have a permanent dipole
C)In compounds that are polar and do not have a permanent dipole
D)In compounds that are nonpolar and that have no permanent dipole

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
17
What is the reason ethylene glycol has a higher boiling point than ethanol?
A)It has a higher molecular mass.
B)It contains more carbon atoms.
C)It has more sites at which to hydrogen bond.
D)It has more dispersion forces.
A)It has a higher molecular mass.
B)It contains more carbon atoms.
C)It has more sites at which to hydrogen bond.
D)It has more dispersion forces.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
18
What do all alkenes have in common?
A)At least one double bond
B)At least one triple bond
C)At least one carbon-carbon bond
D)Carbon-hydrogen bonds
A)At least one double bond
B)At least one triple bond
C)At least one carbon-carbon bond
D)Carbon-hydrogen bonds

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
19
In an alkene with four or more carbon atoms in a row, the double bond can possibly be positioned in more than one location. This is an example of what?
A)Telemores
B)Isomers
C)Ionomers
D)None of the above.
A)Telemores
B)Isomers
C)Ionomers
D)None of the above.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
20
Which is the correct structure for propene?
A)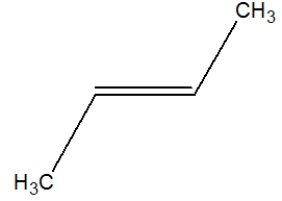
B)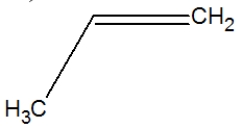
C)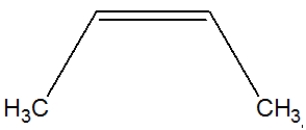
D)Both A and C, above.
A)

B)

C)

D)Both A and C, above.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
21
What is the condensed structural formula for 2-hexane?
A)CH3CH2CH=CHCH2CH3
B)CH2=CH2CH2CH2CH2CH3
C)CH3CH=CHCH2CH2CH3
D)CH3CH=CHCH=CH2CH3
A)CH3CH2CH=CHCH2CH3
B)CH2=CH2CH2CH2CH2CH3
C)CH3CH=CHCH2CH2CH3
D)CH3CH=CHCH=CH2CH3

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
22
What is the name and condensed structural formula of the simplest alkene?
A)Ethene, CH2=CH2
B)Ethane, CH2=CH2
C)Ethene, CH3CH3
D)Ethane, CH3CH3
A)Ethene, CH2=CH2
B)Ethane, CH2=CH2
C)Ethene, CH3CH3
D)Ethane, CH3CH3

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
23
What is the proper Lewis line structure for 3-hexene?
A)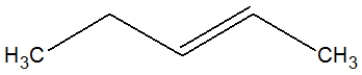
B)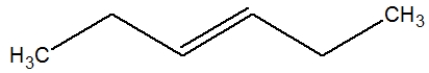
C)
D)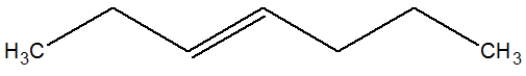
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
24
What is the correct line structure for 3-octene?
A)
B)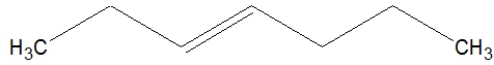
C)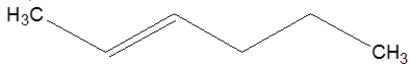
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
25
Compounds with a triple bond, alkynes, all share a general molecular formula. What is it?
A)CnH2n-4
B)CnH2n+2
C)CnH2n
D)CnH2n-2
A)CnH2n-4
B)CnH2n+2
C)CnH2n
D)CnH2n-2

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
26
What is the correct condensed structural formula for 1-hexyne?
A)CHCCHCHCHCH3
B)CH3CH2CCCH2CH3
C)CH2CHCH2CH2CH2CH3
D)CHCCH2CH2CH2CH3
A)CHCCHCHCHCH3
B)CH3CH2CCCH2CH3
C)CH2CHCH2CH2CH2CH3
D)CHCCH2CH2CH2CH3

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
27
Which is the best depiction of the Lewis line structure of 2-hexyne?
A)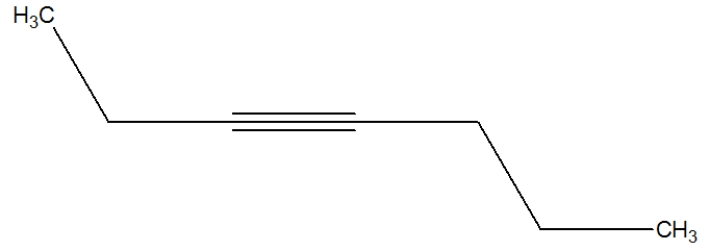
B)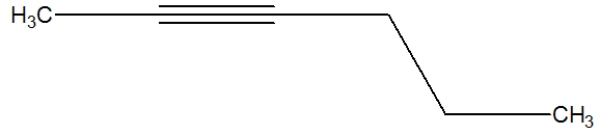
C)
D)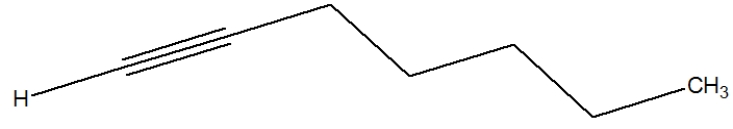
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
28
What is the correct line structure of 1-butyne?
A)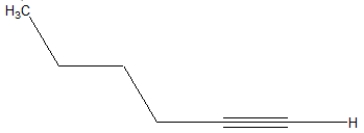
B)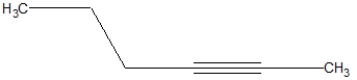
C)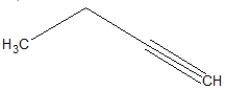
D)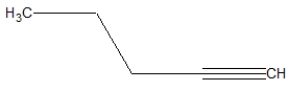
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
29
How many times less soluble in water is oxygen than hydrogen?
A)Four times
B)Two times
C)Their solubility is about the same.
D)None of the above; oxygen is actually more soluble than hydrogen in water.
A)Four times
B)Two times
C)Their solubility is about the same.
D)None of the above; oxygen is actually more soluble than hydrogen in water.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
30
How many isomers can be made from the molecular formula C4H10?
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
31
What is the first rule of naming branched isomers?
A)Count the number of methyl branches
B)Find the longest branch
C)Find the number of branches
D)Find the longest continuous carbon chain
A)Count the number of methyl branches
B)Find the longest branch
C)Find the number of branches
D)Find the longest continuous carbon chain

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
32
What is the name of the following?
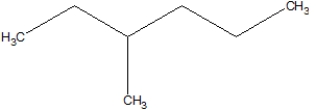
A)2-ethylpentane
B)3-methylhexane
C)2-propylbutane
D)2-methyhexane

A)2-ethylpentane
B)3-methylhexane
C)2-propylbutane
D)2-methyhexane

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
33
The following compound goes by what name?
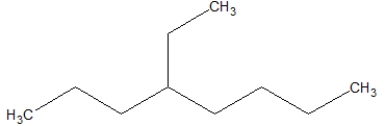
A)4-ethyloctane
B)5-ethyloctane
C)3-propylheptane
D)4-propylheptane

A)4-ethyloctane
B)5-ethyloctane
C)3-propylheptane
D)4-propylheptane

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
34
What is the name of the molecule represented by the condensed structural formula: CH3CH2CHCH3CH3?
A)1-methylbutane
B)2-methylbutane
C)3-methylbutane
D)Dimethylpropane
A)1-methylbutane
B)2-methylbutane
C)3-methylbutane
D)Dimethylpropane

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
35
The molecule 3-methyloctane is represented by what condensed structural formula?
A)CH3CH2CHCH3CH2CH2CH2CH2CH3
B)CH3CH2CH2CH2CH2CH2CH2CH2CH3
C)CH3CH2CH2CHCH3CH2CH2CH2CH3
D)CH3CH2CH2CH3CH2CH2CH2CH2CH3
A)CH3CH2CHCH3CH2CH2CH2CH2CH3
B)CH3CH2CH2CH2CH2CH2CH2CH2CH3
C)CH3CH2CH2CHCH3CH2CH2CH2CH3
D)CH3CH2CH2CH3CH2CH2CH2CH2CH3

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
36
What is the correct line drawing for 3-ethyloctane?
A)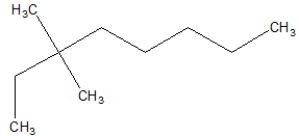
B)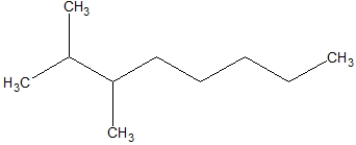
C)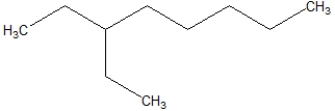
D)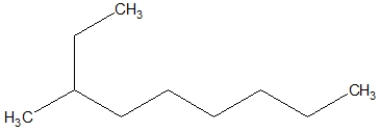
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
37
What is the proper name of this compound?
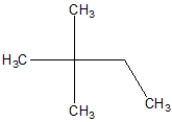
A)2,2-ethylbutane
B)2,2-dimethylpentane
C)2,2-dimethylbutane
D)3,3-methylbutane

A)2,2-ethylbutane
B)2,2-dimethylpentane
C)2,2-dimethylbutane
D)3,3-methylbutane

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
38
What is the correct line structure of 2,2,3,3,-tetramethylbutane, also known as isooctane?
A)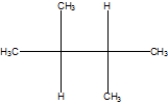
B)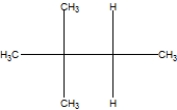
C)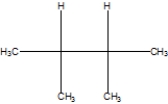
D)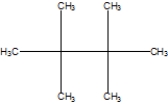
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
39
What is separated in the process of dissolution?
A)Water molecules
B)Ionic solids in water
C)Ions and water
D)Solvent molecules
A)Water molecules
B)Ionic solids in water
C)Ions and water
D)Solvent molecules

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
40
What forces must be overcome for an ionic material to dissolve in water?
A)Water's hydrogen bonding
B)Induced dipole attractions
C)Dispersion forces
D)Ionic electrostatic attractions
A)Water's hydrogen bonding
B)Induced dipole attractions
C)Dispersion forces
D)Ionic electrostatic attractions

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
41
Cyclic alkanes have what general formula?
A)CnHn
B)CnH2n
C)CnH2n-2
D)CnH2n+2
A)CnHn
B)CnH2n
C)CnH2n-2
D)CnH2n+2

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
42
Cylohexane has what molecular formula?
A)C6H14
B)C6H12
C)C6H16
D)C6H13
A)C6H14
B)C6H12
C)C6H16
D)C6H13

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
43
What is the proper molecular formula for cyclooctane, an organic solvent?
A)C8H18
B)C8H16
C)C8H20
D)C8H14
A)C8H18
B)C8H16
C)C8H20
D)C8H14

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
44
What is the name of the following compound?
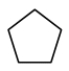
A)Cyclopentane
B)Cyclobutane
C)Cyclohexane
D)Cyclooctane

A)Cyclopentane
B)Cyclobutane
C)Cyclohexane
D)Cyclooctane

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
45
What is the proper line structure for cyclooctane?
A)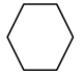
B)
C)
D)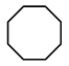
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
46
Alternating double and single bonds in a six-membered carbon ring make a special kind of cyclo-compound. What is its name?
A)Cyclohexane
B)Cyclobenzene
C)Benzene
D)Benzylcyclane
A)Cyclohexane
B)Cyclobenzene
C)Benzene
D)Benzylcyclane

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
47
From what does the term "aromatic" derive, when discussing organic compounds?
A)Early organic compounds that were fragrant were all grouped together and called aromatic.
B)Early inorganic compounds that were fragrant were all grouped together and called aromatic.
C)Early organic compounds that had a stench were all grouped together and called aromatic.
D)Early inorganic compounds that had a stench were all grouped together and called aromatic.
A)Early organic compounds that were fragrant were all grouped together and called aromatic.
B)Early inorganic compounds that were fragrant were all grouped together and called aromatic.
C)Early organic compounds that had a stench were all grouped together and called aromatic.
D)Early inorganic compounds that had a stench were all grouped together and called aromatic.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
48
What is the name of a cyclohexane molecule when one of the carbon atoms in the ring also has a -CH3 attached to it?
A)Cycloheptane
B)Methylcyclohexane
C)Methylcycloheptane
D)Cyclohexane
A)Cycloheptane
B)Methylcyclohexane
C)Methylcycloheptane
D)Cyclohexane

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
49
What is the molecular formula for phenol?
A)C5H6O
B)C6H6O
C)C6H5O
D)C6H6O2
A)C5H6O
B)C6H6O
C)C6H5O
D)C6H6O2

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
50
Temperature of a system seldom influences the rate at which a solute dissolves.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
51
What factors affect the rate of dissolution of a solute in water?
A)The amount of solute already in solution
B)Surface area of the solute
C)Temperature of the solvent
D)All of the above
A)The amount of solute already in solution
B)Surface area of the solute
C)Temperature of the solvent
D)All of the above

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
52
What do all ethers have in common?
A)An oxygen atom connected to two different carbon atoms by single bonds
B)An oxygen atom connected to two different carbon atoms by single or double bonds
C)An oxygen atom connected to one or two different carbon atoms by single bonds
D)An oxygen atom connected to one or two different carbon atoms by single or double bonds
A)An oxygen atom connected to two different carbon atoms by single bonds
B)An oxygen atom connected to two different carbon atoms by single or double bonds
C)An oxygen atom connected to one or two different carbon atoms by single bonds
D)An oxygen atom connected to one or two different carbon atoms by single or double bonds

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
53
What is the correct condensed structural formula for methoxyethane?
A)CH3CH2OCH2CH3
B)CH3OCH2CH3
C)CH3OCH3
D)CH3OCH2CH2CH3
A)CH3CH2OCH2CH3
B)CH3OCH2CH3
C)CH3OCH3
D)CH3OCH2CH2CH3

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
54
What is the name of the following compound?
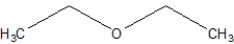
A)Ethoxymethane
B)Methoxymethane
C)Methoxyethane
D)Ethoxyethane

A)Ethoxymethane
B)Methoxymethane
C)Methoxyethane
D)Ethoxyethane

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following is ethoxyethane, also known as diethyl ether?
A)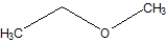
B)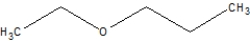
C)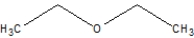
D)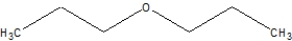
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
56
What is the name of the following compound?
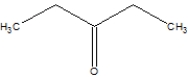
A)3-pentanone
B)3-butanone
C)2-pentanone
D)2-butanone

A)3-pentanone
B)3-butanone
C)2-pentanone
D)2-butanone

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following is 2-hexanone?
A)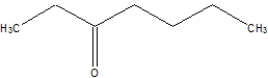
B)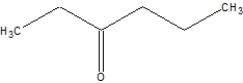
C)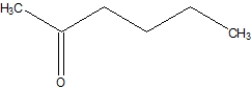
D)None of the above
A)

B)

C)

D)None of the above

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
58
How do the boiling points of ketones relate to those of comparable ethers?
A)They are roughly the same.
B)They are generally lower.
C)They are generally higher.
D)There is no direct relationship.
A)They are roughly the same.
B)They are generally lower.
C)They are generally higher.
D)There is no direct relationship.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
59
How does the ester functional group differ from the ketone group?
A)Ketones have a second oxygen, doubly bonded to the ester carbon and another carbon.
B)Esters have a second oxygen, doubly bonded to the keto carbon and another carbon.
C)Ketones have a second oxygen, singly bonded to the ester carbon and another carbon.
D)Esters have a second oxygen, singly bonded to the keto carbon and another carbon.
A)Ketones have a second oxygen, doubly bonded to the ester carbon and another carbon.
B)Esters have a second oxygen, doubly bonded to the keto carbon and another carbon.
C)Ketones have a second oxygen, singly bonded to the ester carbon and another carbon.
D)Esters have a second oxygen, singly bonded to the keto carbon and another carbon.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
60
What is the name of the following compound?
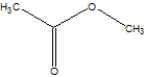
A)Ethylethanoate
B)Methylethanoate
C)Ethylmethanoate
D)Methylmethanoate

A)Ethylethanoate
B)Methylethanoate
C)Ethylmethanoate
D)Methylmethanoate

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
61
Which is the correct structure for ethylethanoate?
A)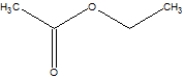
B)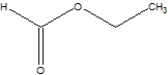
C)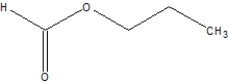
D)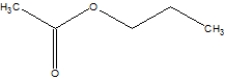
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
62
The term "vapor" means a solute or solvent in a gas phase.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
63
What is the molecular cause of vapor pressure?
A)Gas particles colliding with the container wall
B)Gas or liquid particles colliding with the container wall
C)The temperature of the container in which a material is housed
D)The temperature of the material in a closed system
A)Gas particles colliding with the container wall
B)Gas or liquid particles colliding with the container wall
C)The temperature of the container in which a material is housed
D)The temperature of the material in a closed system

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
64
What does the term "primary amine" denote?
A)An organic compound in which a nitrogen atom is bonded to one hydrogen atom and two carbon atoms
B)An organic compound in which a nitrogen atom is bonded to one carbon atom and two hydrogen atoms
C)An organic compound in which a nitrogen atom is bonded to one hetero atom and two hydrogen atoms
D)An organic compound in which a nitrogen atom is bonded to one hydrogen atom and two hetero atoms
A)An organic compound in which a nitrogen atom is bonded to one hydrogen atom and two carbon atoms
B)An organic compound in which a nitrogen atom is bonded to one carbon atom and two hydrogen atoms
C)An organic compound in which a nitrogen atom is bonded to one hetero atom and two hydrogen atoms
D)An organic compound in which a nitrogen atom is bonded to one hydrogen atom and two hetero atoms

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
65
Which is the correct line structure for butylamine?
A)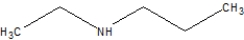
B)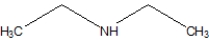
C)
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following is a secondary amine?
A)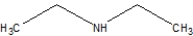
B)
C)
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following is not a primary amine?
A)CH3CH2CH2NH2
B)CH3NHCH2CH3
C)CH3CH2NH2
D)NH2CH2CH2CH2CH3
A)CH3CH2CH2NH2
B)CH3NHCH2CH3
C)CH3CH2NH2
D)NH2CH2CH2CH2CH3

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
68
What is the name of the following?

A)Butylethylamine
B)Propylethylamine
C)Butylpropylamine
D)Propylpropylamine

A)Butylethylamine
B)Propylethylamine
C)Butylpropylamine
D)Propylpropylamine

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
69
What is the name for the following compound: CH3CH2NHCH2CH2CH3?
A)Ethylpropylamine
B)Propylethylamine
C)Ethylbutylamine
D)Propylbutylamine
A)Ethylpropylamine
B)Propylethylamine
C)Ethylbutylamine
D)Propylbutylamine

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
70
How is the boiling point of a solution different than that of a pure solvent or pure liquid?
A)It is lower.
B)It is higher.
C)It remains unchanged.
D)It is changed only if the solute is an ionic material.
A)It is lower.
B)It is higher.
C)It remains unchanged.
D)It is changed only if the solute is an ionic material.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
71
What variable is used in computing molality that is not used in computing molarity?
A)Liters of solution
B)Kilograms of solution
C)Moles of solute
D)None of the above
A)Liters of solution
B)Kilograms of solution
C)Moles of solute
D)None of the above

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
72
If a student knows the mass of a solute and the volume of the solvent, what else must be known to compute the molality of the solution?
A)The density of the solvent
B)The temperature of the solvent
C)The volume of the solute
D)The size of the container
A)The density of the solvent
B)The temperature of the solvent
C)The volume of the solute
D)The size of the container

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
73
What do all alcohols and alcohol congeners have in common?
A)The C-O-C functional group
B)The =O functional group
C)The -OH functional group
D)Nothing; there are too many to have one common functional group.
A)The C-O-C functional group
B)The =O functional group
C)The -OH functional group
D)Nothing; there are too many to have one common functional group.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
74
What is the structural formula for 1-butanol?
A)CH2CH3CH2CH2OH
B)CH3CH3OH
C)CH3CH2CH2OH
D)CH3CH2CH2CH2OH
A)CH2CH3CH2CH2OH
B)CH3CH3OH
C)CH3CH2CH2OH
D)CH3CH2CH2CH2OH

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
75
What is the name of the following compound?

A)2-butanol
B)3-butanol
C)2-pentanol
D)1-pentanol

A)2-butanol
B)3-butanol
C)2-pentanol
D)1-pentanol

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
76
What is the name of the following compound?
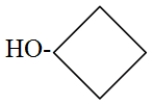
A)1-Butanol
B)Cyclobutanol
C)2-Butanol
D)2-Cyclobutanol

A)1-Butanol
B)Cyclobutanol
C)2-Butanol
D)2-Cyclobutanol

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
77
What does the functional group R-OH indicate?
A)An ether
B)An ester
C)An alcohol
D)An amine
A)An ether
B)An ester
C)An alcohol
D)An amine

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
78
What do all aldehydes have in common?
A)The functional group R-(C=O)H
B)The functional group R-OH
C)The functional group R-NH2
D)The functional group R-O-R
A)The functional group R-(C=O)H
B)The functional group R-OH
C)The functional group R-NH2
D)The functional group R-O-R

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
79
What is the correct line structure for butanal?
A)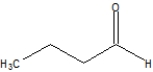
B)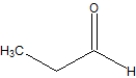
C)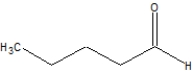
D)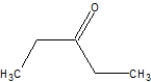
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
80
Why are hangovers more severe when they are induced by alcoholic beverages with high alcohol-congener levels?
A)The congeners are water-soluble, and thus remain in the body a shorter period of time.
B)The congeners are fat-soluble, and thus remain in the body a shorter period of time.
C)The congeners are water-soluble, and thus remain in the body longer.
D)The congeners are fat-soluble, and thus remain in the body longer.
A)The congeners are water-soluble, and thus remain in the body a shorter period of time.
B)The congeners are fat-soluble, and thus remain in the body a shorter period of time.
C)The congeners are water-soluble, and thus remain in the body longer.
D)The congeners are fat-soluble, and thus remain in the body longer.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck


