Exam 7: Aqueous Solutions: Part II
Exam 1: Understanding Our World With Chemistry129 Questions
Exam 2: Matter: Properties, Changes and Measurements126 Questions
Exam 3: Understanding Atoms120 Questions
Exam 4: Bonding and Reactions112 Questions
Exam 5: Chemistry of Bonding: Structure and Function of Drug Molecules123 Questions
Exam 6: Aqueous Solutions: Part I118 Questions
Exam 7: Aqueous Solutions: Part II122 Questions
Exam 8: Organic Chemistry and Polymers123 Questions
Exam 9: Chemistry of Fire and Heat122 Questions
Exam 10: Chemistry of Explosions124 Questions
Exam 11: Applications of Chemical Kinetics124 Questions
Exam 12: Nuclear Chemistry: Energy, Medicine, Weapons, and Terrorism122 Questions
Exam 13: Chemical Equilibrium125 Questions
Exam 14: Introduction to Biochemistry118 Questions
Select questions type
What is the name of the following compound?
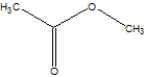
Free
(Multiple Choice)
4.8/5  (33)
(33)
Correct Answer:
B
Of the following, which best represents octane?
Free
(Multiple Choice)
4.8/5  (40)
(40)
Correct Answer:
A
What compound is represented by the following condensed structural formula: CH3CH2COOH?
(Short Answer)
4.7/5  (30)
(30)
Carboxylic acids are usually represented by what general formula?
(Short Answer)
4.9/5  (40)
(40)
What is the name and condensed structural formula of the simplest alkene?
(Multiple Choice)
4.8/5  (36)
(36)
From what sources were organic chemicals originally isolated, until the early nineteenth century?
(Short Answer)
4.9/5  (25)
(25)
What compounds will come out of an HPLC column first, when the stationary phase is coated with an amino-type compound?
(Multiple Choice)
4.8/5  (39)
(39)
What is the reason ethylene glycol has a higher boiling point than ethanol?
(Multiple Choice)
4.8/5  (36)
(36)
Showing 1 - 20 of 122
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)