Deck 27: P-Block Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/33
Play
Full screen (f)
Deck 27: P-Block Chemistry
1
The elements of Group 16 are a mixture of metals, non metals and metalloids.
True
2
Oxidation states in covalently bonded compounds are often referred to as ______ oxidation states.
formal
3
Which of the following oxidation states are common for Group 17 elements? Please select all that apply.
A) +7
B) +1
C) -1
D) +4
A) +7
B) +1
C) -1
D) +4
A, B, C
4
Match the p-block oxide with the expected structure bearing in mind that covalent compounds are formed from elements of similar electronegativity by electron sharing.
-SO2
A) Covalent molecules
B) Covalent network with polar bonds
C) Covalent molecules
D) Ionic structure showing some covalency
-SO2
A) Covalent molecules
B) Covalent network with polar bonds
C) Covalent molecules
D) Ionic structure showing some covalency

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
5
Match the p-block oxide with the expected structure bearing in mind that covalent compounds are formed from elements of similar electronegativity by electron sharing.
-SiO2
A) Covalent molecules
B) Covalent network with polar bonds
C) Covalent molecules
D) Ionic structure showing some covalency
-SiO2
A) Covalent molecules
B) Covalent network with polar bonds
C) Covalent molecules
D) Ionic structure showing some covalency

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
6
Match the p-block oxide with the expected structure bearing in mind that covalent compounds are formed from elements of similar electronegativity by electron sharing.
-XeO4
A) Covalent molecules
B) Covalent network with polar bonds
C) Covalent molecules
D) Ionic structure showing some covalency
-XeO4
A) Covalent molecules
B) Covalent network with polar bonds
C) Covalent molecules
D) Ionic structure showing some covalency

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
7
Match the p-block oxide with the expected structure bearing in mind that covalent compounds are formed from elements of similar electronegativity by electron sharing.
-Ga2O3
A) Covalent molecules
B) Covalent network with polar bonds
C) Covalent molecules
D) Ionic structure showing some covalency
-Ga2O3
A) Covalent molecules
B) Covalent network with polar bonds
C) Covalent molecules
D) Ionic structure showing some covalency

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following statements apply to the p-block elements? Please select all that apply.
A) The oxidation state of the common anions is always equal to the number of valence electrons minus eight.
B) The ionic character decreases left to right across the period.
C) The acidic character increases left to right across the period.
D) The maximum oxidation state of the cation, increases left to right across the Periodic Table.
A) The oxidation state of the common anions is always equal to the number of valence electrons minus eight.
B) The ionic character decreases left to right across the period.
C) The acidic character increases left to right across the period.
D) The maximum oxidation state of the cation, increases left to right across the Periodic Table.

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
9
The incidence of the oxidation state two less than the group number in the p-block elements _______ down the group.

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
10
Bond enthalpies get smaller for M-X as a group in the p block is descended.

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
11
In the third period (Na to Cl), the M-X bond when X has lone pairs, is always stronger than the M-X bond of the corresponding element in the row above. Please select all that apply.
A) Due to a short bond length, the lone pair repulsion between M and X is stronger for the first row.
B) Due to a short bond length in the first row, the electron rich ligands with lone pairs are brought close together and repel each other.
C) The bigger difference in electronegativity between the two species results in polarization of the bonds and more ionic character for the second row.
D) The bigger difference in electronegativity between the two species results in polarisation of the bonds and less ionic character for the second row.
A) Due to a short bond length, the lone pair repulsion between M and X is stronger for the first row.
B) Due to a short bond length in the first row, the electron rich ligands with lone pairs are brought close together and repel each other.
C) The bigger difference in electronegativity between the two species results in polarization of the bonds and more ionic character for the second row.
D) The bigger difference in electronegativity between the two species results in polarisation of the bonds and less ionic character for the second row.

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
12
Gallium lies in Group 13 of the Period Table but a compound with empirical formula 'GaCl2' exists - how is this possible?
A) Group 13 elements form +3 compounds and +2 compounds.
B) The compound is exhibiting the inert pair effect forming Ga+ and Cl2- ions.
C) The compound is exhibiting the inert pair effect and contains both Ga(I) and Ga(III) ions i.e. Ga+[GaCl4]-.
D) It forms a structure similar to aluminium chloride and is made up of three centre, four electron bonds.
A) Group 13 elements form +3 compounds and +2 compounds.
B) The compound is exhibiting the inert pair effect forming Ga+ and Cl2- ions.
C) The compound is exhibiting the inert pair effect and contains both Ga(I) and Ga(III) ions i.e. Ga+[GaCl4]-.
D) It forms a structure similar to aluminium chloride and is made up of three centre, four electron bonds.

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
13
Unlike iron, aluminium is naturally resistant to corrosion as a thin layer of oxide forms on the surface

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
14
Why is the C-F bond significantly stronger than either the C-C or the F-F bond? Please select all that apply.
A) C-F is strengthened by electrostatic interactions.
B) C-C is very weak due to a number of lone pairs.
C) F-F is very weak due to a number of lone pairs and a very short bond.
D) Bonds between atoms that are different tend to be stronger than bonds between atoms that are the same
A) C-F is strengthened by electrostatic interactions.
B) C-C is very weak due to a number of lone pairs.
C) F-F is very weak due to a number of lone pairs and a very short bond.
D) Bonds between atoms that are different tend to be stronger than bonds between atoms that are the same

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
15
BI3 is a much stronger Lewis acid than BF3.

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
16
An enthalpy cycle for the formation of monovalent aluminium halides is given below.
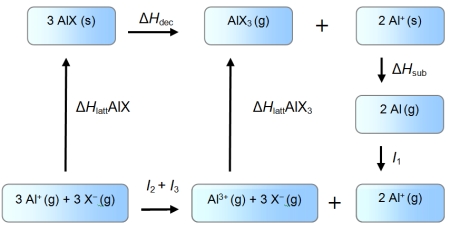
Use the data given below (kJ mol-1) to show which oxidation state is favoured in each case.
I1 = 577 kJ mol-1 I2 = 1816 kJ mol-1 I3 = 2743 kJ mol-1
ΔHsub = 324 kJ mol-1
ΔH (AlF) = -910 kJ mol-1
ΔH (AlI) = -696 kJ mol-1
ΔH (AlF3) = -6380 kJ mol-1
ΔH (AlI3)= -4706 kJ mol-1
A) aluminium(III) fluoride and aluminium(III) iodide
B) aluminium(I) fluoride and aluminium(III) iodide
C) aluminium(I) fluoride and aluminium(I) iodide
D) aluminium(III) fluoride and aluminium(I) iodide

Use the data given below (kJ mol-1) to show which oxidation state is favoured in each case.
I1 = 577 kJ mol-1 I2 = 1816 kJ mol-1 I3 = 2743 kJ mol-1
ΔHsub = 324 kJ mol-1
ΔH (AlF) = -910 kJ mol-1
ΔH (AlI) = -696 kJ mol-1
ΔH (AlF3) = -6380 kJ mol-1
ΔH (AlI3)= -4706 kJ mol-1
A) aluminium(III) fluoride and aluminium(III) iodide
B) aluminium(I) fluoride and aluminium(III) iodide
C) aluminium(I) fluoride and aluminium(I) iodide
D) aluminium(III) fluoride and aluminium(I) iodide

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
17
The bridging chlorine atoms in the dimer Al2Cl6 are involved in 3-centre 4-electron bonds.

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
18
The most abundant oxides of carbon and silicon have totally different structures. Carbon dioxide is a triatomic gas and silicon dioxide forms a macromolecular solid. The difference in structure of the two compounds is due to the difference in bond strength.

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following species does not act as a Lewis Acid?
A) SiF4
B) SnI4
C) CCl4
D) SiCl4
A) SiF4
B) SnI4
C) CCl4
D) SiCl4

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
20
Cyanogen, (CN)2, is sometimes called a pseudohalogen. Why? Please select all that apply:
A) It exists as a dimer.
B) It forms similar compounds to the halogens, e.g. Cl- and CN- both give a white precipitate with silver nitrate.
C) The bond between the CN-CN groups can be broken by UV light.
D) It reacts with halogens readily.
A) It exists as a dimer.
B) It forms similar compounds to the halogens, e.g. Cl- and CN- both give a white precipitate with silver nitrate.
C) The bond between the CN-CN groups can be broken by UV light.
D) It reacts with halogens readily.

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
21
KClO3 and S are used in the heads of safety matches. The reaction between them (which is extremely exothermic) is initiated by friction caused by striking the head of the match against a strip of white phosphorus.

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
22
Match the equation with the type of reaction occurring.
-O22- → O2_ + 1/2O2
A) disproportionation
B) catenation
C) conproportionation
D) deliquescence
-O22- → O2_ + 1/2O2
A) disproportionation
B) catenation
C) conproportionation
D) deliquescence

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
23
Match the equation with the type of reaction occurring.
-I3- + I2 → I5-
A) disproportionation
B) catenation
C) conproportionation
D) deliquescence
-I3- + I2 → I5-
A) disproportionation
B) catenation
C) conproportionation
D) deliquescence

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
24
Match the equation with the type of reaction occurring.
-NH4NO3 (s) → N2O (g) + 2H2O (g)
A) disproportionation
B) catenation
C) conproportionation
D) deliquescence
-NH4NO3 (s) → N2O (g) + 2H2O (g)
A) disproportionation
B) catenation
C) conproportionation
D) deliquescence

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
25
Match the equation with the type of reaction occurring.
-Cu(NO3)2·5H2O (s) → Cu(NO3)2 (aq)
A) disproportionation
B) catenation
C) conproportionation
D) deliquescence
-Cu(NO3)2·5H2O (s) → Cu(NO3)2 (aq)
A) disproportionation
B) catenation
C) conproportionation
D) deliquescence

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
26
Chlorofluorocarbons are responsible for depleting stratospheric ozone. Fluorine radicals react with ozone to generate oxygen and regenerate the fluorine radicals.

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
27
The bond dissociation enthalpies of the Group 17 elements in reducing order of magnitude is?
A) F-F > Cl-C l >Br-Br > I-I > As-As
B) Cl-Cl > Br-Br > F-F > I-I > As-As
C) Cl-Cl > F-F > Br-Br > I-I > As-As
D) As-As > I-I > Br-Br > Cl-Cl > F-F
A) F-F > Cl-C l >Br-Br > I-I > As-As
B) Cl-Cl > Br-Br > F-F > I-I > As-As
C) Cl-Cl > F-F > Br-Br > I-I > As-As
D) As-As > I-I > Br-Br > Cl-Cl > F-F

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
28
Sulfur hexafluoride is very unreactive because the sulfur centre is extremely sterically hindered.

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
29
Match the number of double bonds with the chlorine containing compounds. (Note: It might help to draw the Lewis structures of each chlorine compound.)
-HClO4
A) 3
B) 2
C) 1
-HClO4
A) 3
B) 2
C) 1

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
30
Match the number of double bonds with the chlorine containing compounds. (Note: It might help to draw the Lewis structures of each chlorine compound.)
-ClO2
A) 3
B) 2
C) 1
-ClO2
A) 3
B) 2
C) 1

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
31
Match the number of double bonds with the chlorine containing compounds. (Note: It might help to draw the Lewis structures of each chlorine compound.)
-HClO2
A) 3
B) 2
C) 1
-HClO2
A) 3
B) 2
C) 1

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
32
ClF5 is an example of an interhalogen.

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
33
Which elements of Group 18 form compounds that exist under standard conditions? Please select all that apply:
A) He
B) Kr
C) Ne
D) Xe
A) He
B) Kr
C) Ne
D) Xe

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck


