Exam 27: P-Block Chemistry
Exam 1: Fundamentals57 Questions
Exam 2: The Language of Organic Chemistry25 Questions
Exam 3: Atomic Structure and Properties34 Questions
Exam 4: Diatomic Molecules27 Questions
Exam 5: Polyatomic Molecules30 Questions
Exam 6: Solids31 Questions
Exam 7: Acids and Bases27 Questions
Exam 8: Gases38 Questions
Exam 9: Reaction Kinetics50 Questions
Exam 10: Molecular Spectroscopy25 Questions
Exam 11: Analytical Chemistry24 Questions
Exam 12: Molecular Characterisation30 Questions
Exam 13: Energy and Thermochemistry47 Questions
Exam 14: Entropy and Gibbs Energy40 Questions
Exam 16: Electrochemistry24 Questions
Exam 17: Phase Equilibrium and Solutions25 Questions
Exam 18: Isomerism and Stereochemistry25 Questions
Exam 19: Organic Reaction Mechanisms28 Questions
Exam 20: Halogenoalkanes: Substitution and Elimination Reactions31 Questions
Exam 21: Alkenes and Alkynes: Electrophilic Addition and Pericyclic Reactions27 Questions
Exam 22: Benzene and Other Aromatic Compounds: Electrophilic Substitution Reactions37 Questions
Exam 23: Aldehydes and Ketones: Nucleophilic Addition and Α-Substitution Reactions33 Questions
Exam 24: Carboxylic Acids and Derivatives: Nucleophilic Acyl Substitution and Α-Substitution Reactions25 Questions
Exam 25: Hydrogen30 Questions
Exam 26: S-Block Chemistry28 Questions
Exam 27: P-Block Chemistry33 Questions
Exam 28: D-Block Chemistry34 Questions
Select questions type
Gallium lies in Group 13 of the Period Table but a compound with empirical formula 'GaCl2' exists - how is this possible?
Free
(Multiple Choice)
4.9/5  (32)
(32)
Correct Answer:
C
Bond enthalpies get smaller for M-X as a group in the p block is descended.
Free
(True/False)
5.0/5  (42)
(42)
Correct Answer:
True
The bridging chlorine atoms in the dimer Al2Cl6 are involved in 3-centre 4-electron bonds.
Free
(True/False)
4.9/5  (35)
(35)
Correct Answer:
True
The elements of Group 16 are a mixture of metals, non metals and metalloids.
(True/False)
4.8/5  (28)
(28)
Match the p-block oxide with the expected structure bearing in mind that covalent compounds are formed from elements of similar electronegativity by electron sharing.
-XeO4
(Multiple Choice)
4.8/5  (26)
(26)
Which elements of Group 18 form compounds that exist under standard conditions? Please select all that apply:
(Multiple Choice)
4.8/5  (33)
(33)
An enthalpy cycle for the formation of monovalent aluminium halides is given below.
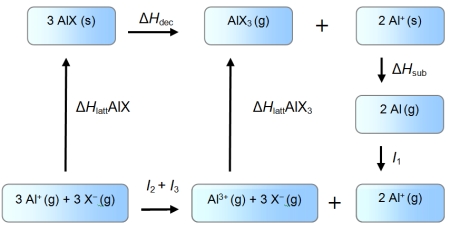 Use the data given below (kJ mol-1) to show which oxidation state is favoured in each case.
I1 = 577 kJ mol-1 I2 = 1816 kJ mol-1 I3 = 2743 kJ mol-1
ΔHsub = 324 kJ mol-1
ΔH (AlF) = -910 kJ mol-1
ΔH (AlI) = -696 kJ mol-1
ΔH (AlF3) = -6380 kJ mol-1
ΔH (AlI3)= -4706 kJ mol-1
Use the data given below (kJ mol-1) to show which oxidation state is favoured in each case.
I1 = 577 kJ mol-1 I2 = 1816 kJ mol-1 I3 = 2743 kJ mol-1
ΔHsub = 324 kJ mol-1
ΔH (AlF) = -910 kJ mol-1
ΔH (AlI) = -696 kJ mol-1
ΔH (AlF3) = -6380 kJ mol-1
ΔH (AlI3)= -4706 kJ mol-1
(Multiple Choice)
4.7/5  (33)
(33)
In the third period (Na to Cl), the M-X bond when X has lone pairs, is always stronger than the M-X bond of the corresponding element in the row above. Please select all that apply.
(Multiple Choice)
4.8/5  (32)
(32)
Match the equation with the type of reaction occurring.
-NH4NO3 (s) → N2O (g) + 2H2O (g)
(Multiple Choice)
4.8/5  (38)
(38)
Match the number of double bonds with the chlorine containing compounds. (Note: It might help to draw the Lewis structures of each chlorine compound.)
-HClO2
(Multiple Choice)
4.9/5  (43)
(43)
Match the p-block oxide with the expected structure bearing in mind that covalent compounds are formed from elements of similar electronegativity by electron sharing.
-Ga2O3
(Multiple Choice)
4.7/5  (37)
(37)
Match the number of double bonds with the chlorine containing compounds. (Note: It might help to draw the Lewis structures of each chlorine compound.)
-ClO2
(Multiple Choice)
4.8/5  (35)
(35)
Chlorofluorocarbons are responsible for depleting stratospheric ozone. Fluorine radicals react with ozone to generate oxygen and regenerate the fluorine radicals.
(True/False)
4.8/5  (36)
(36)
Match the p-block oxide with the expected structure bearing in mind that covalent compounds are formed from elements of similar electronegativity by electron sharing.
-SiO2
(Multiple Choice)
4.9/5  (35)
(35)
Oxidation states in covalently bonded compounds are often referred to as ______ oxidation states.
(Short Answer)
4.7/5  (27)
(27)
Why is the C-F bond significantly stronger than either the C-C or the F-F bond? Please select all that apply.
(Multiple Choice)
4.7/5  (32)
(32)
Match the equation with the type of reaction occurring.
-I3- + I2 → I5-
(Multiple Choice)
4.8/5  (30)
(30)
The bond dissociation enthalpies of the Group 17 elements in reducing order of magnitude is?
(Multiple Choice)
4.8/5  (36)
(36)
Match the equation with the type of reaction occurring.
-Cu(NO3)2·5H2O (s) → Cu(NO3)2 (aq)
(Multiple Choice)
4.9/5  (33)
(33)
Showing 1 - 20 of 33
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)