Deck 14: Entropy and Gibbs Energy
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/40
Play
Full screen (f)
Deck 14: Entropy and Gibbs Energy
1
The following substances are ordered with increasing entropy: neon gas < liquid water < a gold bar.
False
2
The entropy change for the following reaction will be negative:
C11H22O11 (s) + 12 O2 (g) → 12 CO2 (g) + 11 H2O (l)
C11H22O11 (s) + 12 O2 (g) → 12 CO2 (g) + 11 H2O (l)
False
3
The entropy change for the following reaction will be positive:
N2 (g) + 3 H2 (g) → 2 NH3 (g)
N2 (g) + 3 H2 (g) → 2 NH3 (g)
False
4
Entropy is identified with the amount of ________ in the system.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
5
Calculate the entropy change ( 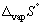 , J K-1 mol-1) when 1.00 mol of ethanol at its boiling point (Tb =78.45 °C) vaporizes (in the process the temperature does not change,
, J K-1 mol-1) when 1.00 mol of ethanol at its boiling point (Tb =78.45 °C) vaporizes (in the process the temperature does not change, 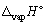 for ethanol is + 43.5 kJ mol-1).
for ethanol is + 43.5 kJ mol-1).
A) + 124.
B) - 124.
C) + 554.
D) + 0.12.
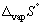 , J K-1 mol-1) when 1.00 mol of ethanol at its boiling point (Tb =78.45 °C) vaporizes (in the process the temperature does not change,
, J K-1 mol-1) when 1.00 mol of ethanol at its boiling point (Tb =78.45 °C) vaporizes (in the process the temperature does not change, 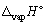 for ethanol is + 43.5 kJ mol-1).
for ethanol is + 43.5 kJ mol-1).A) + 124.
B) - 124.
C) + 554.
D) + 0.12.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
6
Calculate the entropy change ( 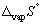 , J K-1 mol-1) when 1.00 mol of mercury at its boiling point (Tb = 356.55 °C) vaporizes (in the process the temperature does not change,
, J K-1 mol-1) when 1.00 mol of mercury at its boiling point (Tb = 356.55 °C) vaporizes (in the process the temperature does not change, 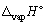 for mercury is + 59.3 kJ mol-1).
for mercury is + 59.3 kJ mol-1).
A) - 94.
B) + 94.
C) + 166.
D) + 0.09.
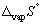 , J K-1 mol-1) when 1.00 mol of mercury at its boiling point (Tb = 356.55 °C) vaporizes (in the process the temperature does not change,
, J K-1 mol-1) when 1.00 mol of mercury at its boiling point (Tb = 356.55 °C) vaporizes (in the process the temperature does not change, 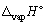 for mercury is + 59.3 kJ mol-1).
for mercury is + 59.3 kJ mol-1).A) - 94.
B) + 94.
C) + 166.
D) + 0.09.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
7
Calculate the entropy change ( 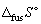 , J K-1 mol-1) when 1.00 mol of methane at its melting point (Tm = - 182.05 °C) freezes (in the process the temperature does not change and
, J K-1 mol-1) when 1.00 mol of methane at its melting point (Tm = - 182.05 °C) freezes (in the process the temperature does not change and 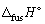 for methane is + 0.94 kJ mol-1).
for methane is + 0.94 kJ mol-1).
A) - 10.3.
B) - 0.01.
C) + 10.3.
D) - 5.2.
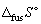 , J K-1 mol-1) when 1.00 mol of methane at its melting point (Tm = - 182.05 °C) freezes (in the process the temperature does not change and
, J K-1 mol-1) when 1.00 mol of methane at its melting point (Tm = - 182.05 °C) freezes (in the process the temperature does not change and 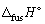 for methane is + 0.94 kJ mol-1).
for methane is + 0.94 kJ mol-1).A) - 10.3.
B) - 0.01.
C) + 10.3.
D) - 5.2.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
8
For water 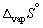 = + 109 J K-1 mol-1 whilst
= + 109 J K-1 mol-1 whilst 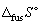 = + 22 J K-1 mol-1. The entropy change for vaporization is larger than the entropy change for fusion because it is measured at a higher temperature.
= + 22 J K-1 mol-1. The entropy change for vaporization is larger than the entropy change for fusion because it is measured at a higher temperature.
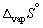 = + 109 J K-1 mol-1 whilst
= + 109 J K-1 mol-1 whilst 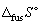 = + 22 J K-1 mol-1. The entropy change for vaporization is larger than the entropy change for fusion because it is measured at a higher temperature.
= + 22 J K-1 mol-1. The entropy change for vaporization is larger than the entropy change for fusion because it is measured at a higher temperature.
Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
9
Whether the total entropy change (ΔS(total)) of a process is positive, negative, or equal to zero defines whether the process is spontaneous. Match the (ΔS(total)) condition with its description.
-ΔS(total) > 0
A) spontaneous process
B) non-spontaneous process
C) process at equilibrium
-ΔS(total) > 0
A) spontaneous process
B) non-spontaneous process
C) process at equilibrium

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
10
Whether the total entropy change (ΔS(total)) of a process is positive, negative, or equal to zero defines whether the process is spontaneous. Match the (ΔS(total)) condition with its description.
-ΔS(total) < 0
A) spontaneous process
B) non-spontaneous process
C) process at equilibrium
-ΔS(total) < 0
A) spontaneous process
B) non-spontaneous process
C) process at equilibrium

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
11
Whether the total entropy change (ΔS(total)) of a process is positive, negative, or equal to zero defines whether the process is spontaneous. Match the (ΔS(total)) condition with its description.
-ΔS(total) = 0
A) spontaneous process
B) non-spontaneous process
C) process at equilibrium
-ΔS(total) = 0
A) spontaneous process
B) non-spontaneous process
C) process at equilibrium

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
12
Calculate the change of entropy (in J K-1 mol-1) when 1 mol of methanol is heated at constant pressure from 5 °C to 35 °C, the molar heat capacity, Cp, of methanol is 81.6 J K-1 mol-1, assume that the molar heat capacity, Cp is constant over this temperature range.
A) + 8.36.
B) - 8.36.
C) - 158.79.
D) + 158.79.
A) + 8.36.
B) - 8.36.
C) - 158.79.
D) + 158.79.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
13
Calculate the change of entropy (in J K-1 mol-1) when 1 mol of ethanol is cooled at constant pressure from 25 °C to 10 °C, the molar heat capacity, Cp, of ethanol is 111.5 J K-1 mol-1 and assume that Cp is constant over this temperature range.
A) + 5.8.
B) - 5.8.
C) - 102.2.
D) + 102.2.
A) + 5.8.
B) - 5.8.
C) - 102.2.
D) + 102.2.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
14
When 1 mol of trichloromethane is heated from 5 °C to 20 °C at constant pressure, the change of entropy is + 6.00 J K-1 mol-1. When the solvent is heated further from 20 °C to 35 °C, the change in entropy will also be + 6.00 J K-1 mol-1.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
15
When 1 mol of water is heated from 10 °C to 25 °C at constant pressure, the change in entropy is + 3.89 J K-1 mol-1. When 1 mol of methanol is heated from 10 °C to 25 °C at constant pressure, the change in entropy will be smaller.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
16
At zero kelvin the entropy of a perfect crystal is ____.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
17
Calculate the standard entropy 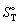 of 1 mol of water at 20 °C and 1 bar. The molar heat capacity, Cp, of water is 75.3 J K-1 mol-1 and
of 1 mol of water at 20 °C and 1 bar. The molar heat capacity, Cp, of water is 75.3 J K-1 mol-1 and 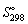 is 69.9 J K-1 mol-1.
is 69.9 J K-1 mol-1.
A) 69.9.
B) - 133.5.
C) 68.7.
D) 71.1.
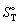 of 1 mol of water at 20 °C and 1 bar. The molar heat capacity, Cp, of water is 75.3 J K-1 mol-1 and
of 1 mol of water at 20 °C and 1 bar. The molar heat capacity, Cp, of water is 75.3 J K-1 mol-1 and 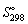 is 69.9 J K-1 mol-1.
is 69.9 J K-1 mol-1.A) 69.9.
B) - 133.5.
C) 68.7.
D) 71.1.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
18
The standard entropy 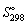 of ethanol, at 1 bar, is 159.9 J K-1 mol-1. At 315 K, its standard entropy will be larger.
of ethanol, at 1 bar, is 159.9 J K-1 mol-1. At 315 K, its standard entropy will be larger.
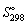 of ethanol, at 1 bar, is 159.9 J K-1 mol-1. At 315 K, its standard entropy will be larger.
of ethanol, at 1 bar, is 159.9 J K-1 mol-1. At 315 K, its standard entropy will be larger.
Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
19
The standard entropy change for the following reaction will be large and positive. CS2 (l) + 3O2 (g) → CO2 (g) + 2SO2 (g)

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
20
Using data in Appendix 7, (p. 1350), calculate the standard entropy change of reaction 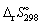 ( in J K-1 mol-1) for the following reaction: CH4 (g) + 2 O2 (g) → CO2 (g) + 2 H2O (l)
( in J K-1 mol-1) for the following reaction: CH4 (g) + 2 O2 (g) → CO2 (g) + 2 H2O (l)
A) + 243.0.
B) - 243.0.
C) - 107.8.
D) + 107.8.
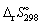 ( in J K-1 mol-1) for the following reaction: CH4 (g) + 2 O2 (g) → CO2 (g) + 2 H2O (l)
( in J K-1 mol-1) for the following reaction: CH4 (g) + 2 O2 (g) → CO2 (g) + 2 H2O (l)A) + 243.0.
B) - 243.0.
C) - 107.8.
D) + 107.8.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
21
Using data in Appendix 7, (p. 1350), calculate the standard entropy change of reaction 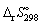 (in J K-1 mol-1) for the following reaction:
(in J K-1 mol-1) for the following reaction:
NH3 (g) + HNO3 (l) → NH4NO3 (s)
A) - 197.
B) + 197.
C) -151.1.
D) +151.1.
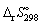 (in J K-1 mol-1) for the following reaction:
(in J K-1 mol-1) for the following reaction:NH3 (g) + HNO3 (l) → NH4NO3 (s)
A) - 197.
B) + 197.
C) -151.1.
D) +151.1.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
22
The standard entropy change, 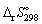 for the following reaction is: -198.7 J K-1 mol-1.
for the following reaction is: -198.7 J K-1 mol-1.
N2 (g) + 3 H2 (g) → 2 NH3 (g) Calculate the standard entropy change of reaction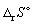 (in J K-1 mol-1) at 550 K.
(in J K-1 mol-1) at 550 K.
A) - 226.
B) - 171.
C) + 226.
D) + 171.
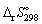 for the following reaction is: -198.7 J K-1 mol-1.
for the following reaction is: -198.7 J K-1 mol-1.N2 (g) + 3 H2 (g) → 2 NH3 (g) Calculate the standard entropy change of reaction
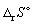 (in J K-1 mol-1) at 550 K.
(in J K-1 mol-1) at 550 K.A) - 226.
B) - 171.
C) + 226.
D) + 171.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
23
The standard entropy change, 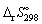 for the following reaction is: + 511.9 J K-1 mol-1.
for the following reaction is: + 511.9 J K-1 mol-1.
C12H22O11 (s) + 12 O2 (g) → 12 CO2 (g) + 11 H2O (l) Calculate the standard entropy change of reaction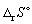 (in J K-1 mol-1) at 390 116.85 °C.
(in J K-1 mol-1) at 390 116.85 °C.
A) + 511.9.
B) + 48.3.
C) + 379.
D) + 645.
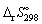 for the following reaction is: + 511.9 J K-1 mol-1.
for the following reaction is: + 511.9 J K-1 mol-1.C12H22O11 (s) + 12 O2 (g) → 12 CO2 (g) + 11 H2O (l) Calculate the standard entropy change of reaction
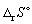 (in J K-1 mol-1) at 390 116.85 °C.
(in J K-1 mol-1) at 390 116.85 °C.A) + 511.9.
B) + 48.3.
C) + 379.
D) + 645.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
24
The standard entropy change, 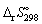 is: - 198.7 J K-1 mol-1, for the following reaction: N2 (g) + 3 H2 (g) → 2 NH3 (g) This violates the Second Law of thermodynamics.
is: - 198.7 J K-1 mol-1, for the following reaction: N2 (g) + 3 H2 (g) → 2 NH3 (g) This violates the Second Law of thermodynamics.
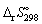 is: - 198.7 J K-1 mol-1, for the following reaction: N2 (g) + 3 H2 (g) → 2 NH3 (g) This violates the Second Law of thermodynamics.
is: - 198.7 J K-1 mol-1, for the following reaction: N2 (g) + 3 H2 (g) → 2 NH3 (g) This violates the Second Law of thermodynamics.
Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
25
Whether Gibbs energy change (ΔG) of a process is positive, negative, or equal to zero defines whether the process is spontaneous. Match the situation with its description.
-ΔG < 0
A) the reaction or process is spontaneous
B) the reaction or process is non-spontaneous
C) the reaction or process is at equilibrium
-ΔG < 0
A) the reaction or process is spontaneous
B) the reaction or process is non-spontaneous
C) the reaction or process is at equilibrium

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
26
Whether Gibbs energy change (ΔG) of a process is positive, negative, or equal to zero defines whether the process is spontaneous. Match the situation with its description.
-ΔG > 0
A) the reaction or process is spontaneous
B) the reaction or process is non-spontaneous
C) the reaction or process is at equilibrium
-ΔG > 0
A) the reaction or process is spontaneous
B) the reaction or process is non-spontaneous
C) the reaction or process is at equilibrium

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
27
Whether Gibbs energy change (ΔG) of a process is positive, negative, or equal to zero defines whether the process is spontaneous. Match the situation with its description.
-ΔG = 0
A) the reaction or process is spontaneous
B) the reaction or process is non-spontaneous
C) the reaction or process is at equilibrium
-ΔG = 0
A) the reaction or process is spontaneous
B) the reaction or process is non-spontaneous
C) the reaction or process is at equilibrium

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
28
Match the reaction description and whether it is spontaneous and under which conditions
-ΔH > 0, ΔS < 0
A) Never spontaneous
B) Spontaneous on heating
C) Always spontaneous
D) Spontaneous on cooling
-ΔH > 0, ΔS < 0
A) Never spontaneous
B) Spontaneous on heating
C) Always spontaneous
D) Spontaneous on cooling

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
29
Match the reaction description and whether it is spontaneous and under which conditions
-ΔH > 0, ΔS > 0
A) Never spontaneous
B) Spontaneous on heating
C) Always spontaneous
D) Spontaneous on cooling
-ΔH > 0, ΔS > 0
A) Never spontaneous
B) Spontaneous on heating
C) Always spontaneous
D) Spontaneous on cooling

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
30
Match the reaction description and whether it is spontaneous and under which conditions
-ΔH < 0, ΔS > 0
A) Never spontaneous
B) Spontaneous on heating
C) Always spontaneous
D) Spontaneous on cooling
-ΔH < 0, ΔS > 0
A) Never spontaneous
B) Spontaneous on heating
C) Always spontaneous
D) Spontaneous on cooling

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
31
Match the reaction description and whether it is spontaneous and under which conditions
-ΔH < 0, ΔS < 0
A) Never spontaneous
B) Spontaneous on heating
C) Always spontaneous
D) Spontaneous on cooling
-ΔH < 0, ΔS < 0
A) Never spontaneous
B) Spontaneous on heating
C) Always spontaneous
D) Spontaneous on cooling

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
32
An endothermic reaction has 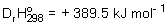 ,
, 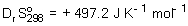 and
and 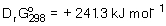 . Find the temperature, T(K), at which the reaction becomes spontaneous.
. Find the temperature, T(K), at which the reaction becomes spontaneous.
A) 298.
B) 783.
C) 0.78.
D) 1.28.
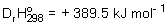 ,
, 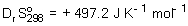 and
and 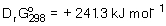 . Find the temperature, T(K), at which the reaction becomes spontaneous.
. Find the temperature, T(K), at which the reaction becomes spontaneous.A) 298.
B) 783.
C) 0.78.
D) 1.28.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
33
Using 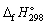 and
and 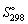 data given below:
data given below:
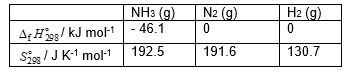
Calculate the standard Gibbs energy change,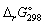 (kJ mol-1), for the following reaction:
(kJ mol-1), for the following reaction:
2 NH3 (g) → N2 (g) + 3 H2 (g)
A) + 151.
B) - 151.
C) + 33.
D) - 59.
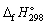 and
and 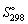 data given below:
data given below:
Calculate the standard Gibbs energy change,
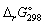 (kJ mol-1), for the following reaction:
(kJ mol-1), for the following reaction:2 NH3 (g) → N2 (g) + 3 H2 (g)
A) + 151.
B) - 151.
C) + 33.
D) - 59.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
34
Using 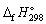 and
and 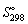 data given below:
data given below:
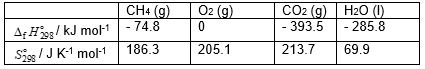 Calculate the standard Gibbs energy change,
Calculate the standard Gibbs energy change, 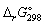 (kJ mol-1), for the following reaction:
(kJ mol-1), for the following reaction:
CH4 (g) + 2 O2 (g) → CO2 (g) + 2 H2O (l)
A) - 818.
B) + 71.5.
C) + 963.
D) - 963.
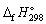 and
and 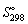 data given below:
data given below: Calculate the standard Gibbs energy change,
Calculate the standard Gibbs energy change, 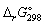 (kJ mol-1), for the following reaction:
(kJ mol-1), for the following reaction:CH4 (g) + 2 O2 (g) → CO2 (g) + 2 H2O (l)
A) - 818.
B) + 71.5.
C) + 963.
D) - 963.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
35
Using 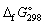 data given below:
data given below:
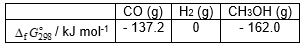
Calculate the standard Gibbs energy change,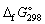 (kJ mol-1), for the following reaction:
(kJ mol-1), for the following reaction:
CO (g) + 2 H2 (g) → CH3OH (g)
A) - 299.2.
B) + 299.2.
C) + 24.8.
D) - 24.8.
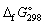 data given below:
data given below:
Calculate the standard Gibbs energy change,
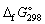 (kJ mol-1), for the following reaction:
(kJ mol-1), for the following reaction:CO (g) + 2 H2 (g) → CH3OH (g)
A) - 299.2.
B) + 299.2.
C) + 24.8.
D) - 24.8.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
36
Using 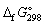 data given below:
data given below:
 calculate the standard Gibbs energy change,
calculate the standard Gibbs energy change, 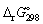 (kJ mol-1), for the following reaction:
(kJ mol-1), for the following reaction:
CO2 (g) + CH4 (g) → CH3CO2H (l)
A) - 835.1
B) - 55.3.
C) + 835.1.
D) + 55.3.
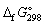 data given below:
data given below: calculate the standard Gibbs energy change,
calculate the standard Gibbs energy change, 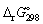 (kJ mol-1), for the following reaction:
(kJ mol-1), for the following reaction:CO2 (g) + CH4 (g) → CH3CO2H (l)
A) - 835.1
B) - 55.3.
C) + 835.1.
D) + 55.3.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
37
Using 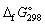 data given below:
data given below:

Calculate the standard Gibbs energy change,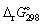 (kJ mol-1), for the following reaction:
(kJ mol-1), for the following reaction:
C12H22O11 (s) + 12 O2 (g) → 12 CO2 (g) + 11 H2O (l)
A) + 911.5.
B) - 5797.9.
C) + 5797.9.
D) - 911.5.
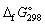 data given below:
data given below:
Calculate the standard Gibbs energy change,
 (kJ mol-1), for the following reaction:
(kJ mol-1), for the following reaction:C12H22O11 (s) + 12 O2 (g) → 12 CO2 (g) + 11 H2O (l)
A) + 911.5.
B) - 5797.9.
C) + 5797.9.
D) - 911.5.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
38
An endothermic reaction with a positive entropy change will become spontaneous on cooling.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
39
Using the data given below estimate the value of the Gibbs energy change of reaction (kJ mol-1) at 310 K, for the following reaction:
CH3CH2OH (l) + O2 (g) → CH3CO2H (l) + H2O (l)
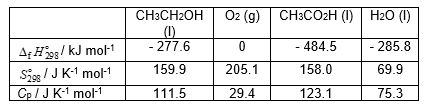
A) - 450.2
B) - 451.8.
C) -41.7.
D) -533.8.
CH3CH2OH (l) + O2 (g) → CH3CO2H (l) + H2O (l)

A) - 450.2
B) - 451.8.
C) -41.7.
D) -533.8.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
40
Using the data given below estimate the value of the Gibbs energy change of reaction at 325 K, for the following reaction:
N2 (g) + 2 O2 (g) → 2 NO2 (g)
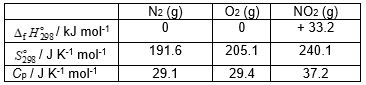
A) + 26.1.
B) + 105.9.
C) + 102.6.
D) + 40.
N2 (g) + 2 O2 (g) → 2 NO2 (g)

A) + 26.1.
B) + 105.9.
C) + 102.6.
D) + 40.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck


