Deck 4: Introduction to Alkenes and Alkynes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/26
Play
Full screen (f)
Deck 4: Introduction to Alkenes and Alkynes
1
Complete the reaction by giving the products. Show all unshared electron pairs and formal charges.
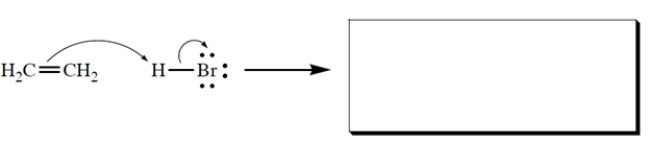

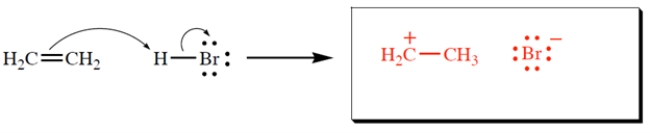
2
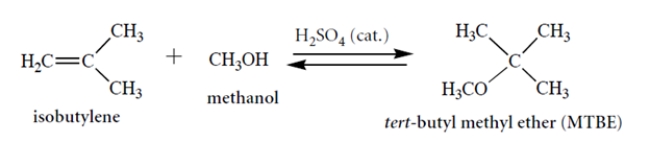
Consider this reaction. Draw a curved-arrow mechanism for the reaction. Notice that concentrated acid is present as a catalyst. (You can abbreviate the catalyst as H-A in your mechanism.) This mechanism should be a series of acid-base steps.

The curved-arrow mechanism is as follows. This is by analogy to the mechanism of HBr addition or the mechanism of hydration, both of which go through a carbocation intermediate.
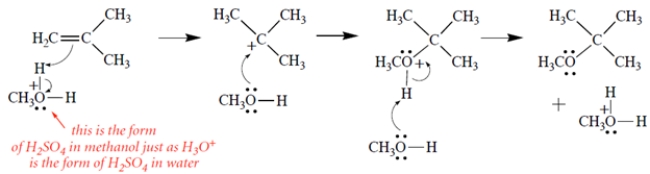 As noted in the problem, you could use H2SO4 as your acid or abbreviate it with H-A. If you ionized the methanol and let the conjugate base attack the carbocation, this is not the expected pathway.
As noted in the problem, you could use H2SO4 as your acid or abbreviate it with H-A. If you ionized the methanol and let the conjugate base attack the carbocation, this is not the expected pathway.
 As noted in the problem, you could use H2SO4 as your acid or abbreviate it with H-A. If you ionized the methanol and let the conjugate base attack the carbocation, this is not the expected pathway.
As noted in the problem, you could use H2SO4 as your acid or abbreviate it with H-A. If you ionized the methanol and let the conjugate base attack the carbocation, this is not the expected pathway. 3
Consider this reaction. Which reaction would be faster: this reaction or the reaction of propene (H2C=CHCH3) with methanol to give isopropyl methyl ether (CH3)2CH-OCH3? Explain. Hammond's postulate should be part of your explanation.
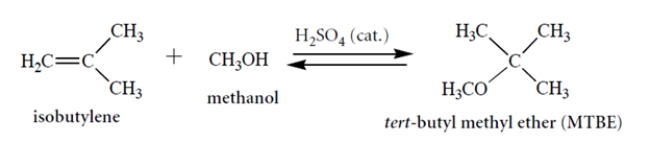

We let the relative energies of the carbocation intermediates guide us and invoke Hammond's postulate, which says that the energies of the transition states should be approximated by the energies of the carbocation intermediates. Hence, the reaction with the more stable carbocation intermediate should be the faster reaction. Because addition to propene involves a secondary carbocation, whereas the reaction above involves a more stable tertiary carbocation, the reaction above is a lot faster.
You must specifically mention the connection between transition states and reactive intermediates (for example, carbocations) for full credit.
You must specifically mention the connection between transition states and reactive intermediates (for example, carbocations) for full credit.
4
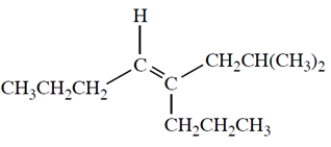
Name the following compound and include the E or Z designation in the name.


Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
5
An alcohol has an empirical formula of C12H20O and a molecular mass of about 360. How many rings plus double bonds does it contain? (Atomic masses: C = 12, H = 1, O = 16)

Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
6
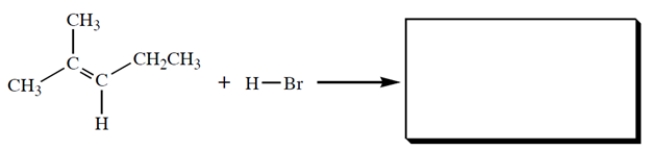
Give the structure of the HBr addition product.


Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
7
Name the compound. Include an E or Z designation if appropriate.
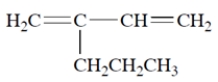
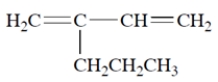

Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
8
Consider this molecule:
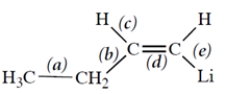 Which bond is an sp2-sp3 bond?
Which bond is an sp2-sp3 bond?
A) bond (a)
B) bond (b)
C) bond (c)
D) bond (d)
E) bond (e)
 Which bond is an sp2-sp3 bond?
Which bond is an sp2-sp3 bond?A) bond (a)
B) bond (b)
C) bond (c)
D) bond (d)
E) bond (e)

Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
9
Provide an IUPAC name for the compound.
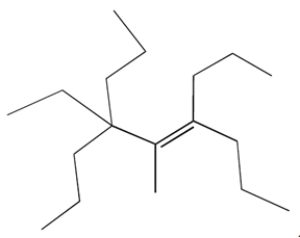


Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
10
Consider the reaction free-energy diagram for two reactions that convert reactants (R) into products (P) and select the two correct statements.
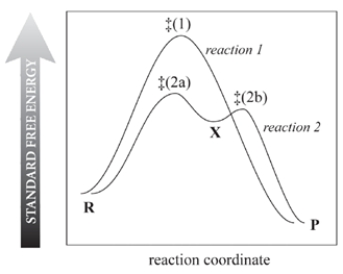
A) Reaction 1 is faster because it occurs in one step.
B) Reaction 2 is faster than reaction 1.
C) Both reactions occur at the same rate at a given concentration of R.
D) X is a transition state of reaction 2.
E) The rate-limiting step of reaction 2 is R ⇌ X.f.
The rate-limiting step of reaction 2 is X ⇌ P.g.
X must be a carbocation.

A) Reaction 1 is faster because it occurs in one step.
B) Reaction 2 is faster than reaction 1.
C) Both reactions occur at the same rate at a given concentration of R.
D) X is a transition state of reaction 2.
E) The rate-limiting step of reaction 2 is R ⇌ X.f.
The rate-limiting step of reaction 2 is X ⇌ P.g.
X must be a carbocation.

Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
11
Give the IUPAC name, including stereochemistry, of the compound:
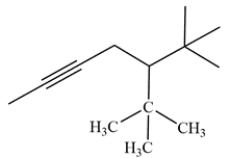


Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
12
Give the structure of the carbocation intermediate and the curved-arrow notation for its formation in the addition reaction of 2-methyl-1-hexene with HBr.
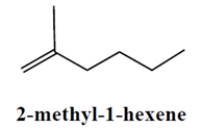


Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
13
Name this compound (including stereochemical configuration where appropriate).
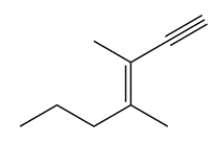


Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
14
14. In each pair, identify the more stable one (as measured by heats of formation).
-1:
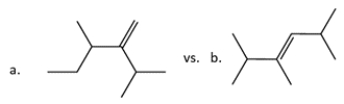 The more stable compound is ________________.
The more stable compound is ________________.
A) Compound a
B) Compound b
-1:
 The more stable compound is ________________.
The more stable compound is ________________.A) Compound a
B) Compound b

Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
15
14. In each pair, identify the more stable one (as measured by heats of formation).
-2:
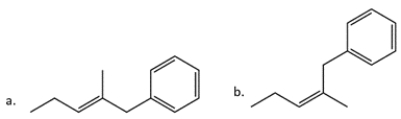 The more stable compound is ________________.
The more stable compound is ________________.
A) Compound a
B) Compound b
-2:
 The more stable compound is ________________.
The more stable compound is ________________.A) Compound a
B) Compound b

Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
16
In this pair of compounds, identify the more stable one (as measured by heats of formation).
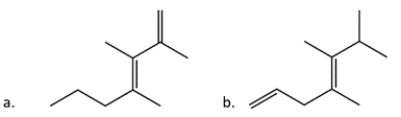
A) Compound a
B) Compound b

A) Compound a
B) Compound b

Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
17
Which of these alkenes has the smallest (or more negative) heat of formation?
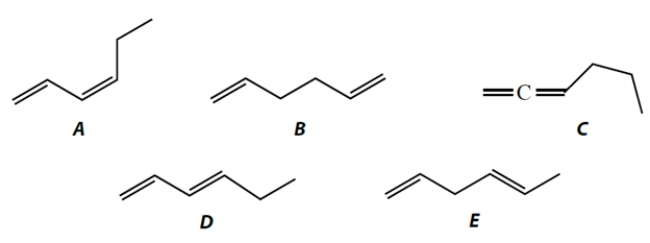
A) Compound A
B) Compound B
C) Compound C
D) Compound D
E) Compound E

A) Compound A
B) Compound B
C) Compound C
D) Compound D
E) Compound E

Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
18
Complete the reaction by giving the major organic product(s).
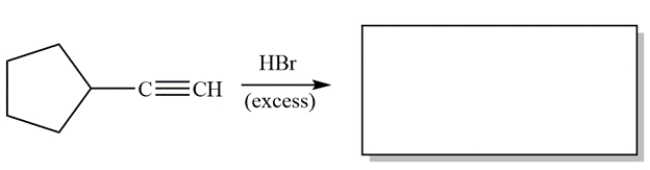


Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
19
Complete the reaction by giving the major organic product(s).
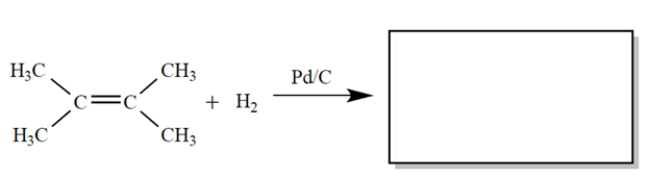


Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
20
Complete the reactions by giving the major organic product.
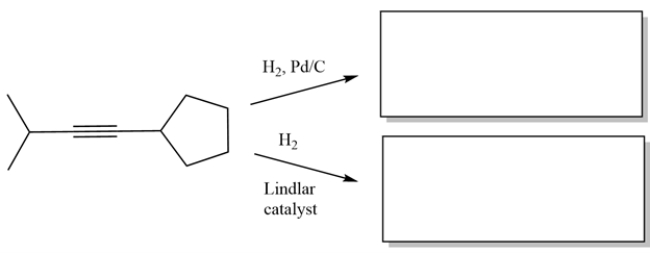


Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
21
Select the true statements that apply to a hydrocarbon, compound X, with the molecular formula C10H14. For purposes of the first two statements, a benzene ring has this skeletal structure:
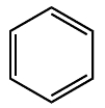
A) Compound X could contain a benzene ring.
B) Compound X must contain a benzene ring.
C) Compound X has an unsaturation number of 8.
D) Compound X has an unsaturation number of 4.
E) Compound X could contain three double bonds and one triple bond.

A) Compound X could contain a benzene ring.
B) Compound X must contain a benzene ring.
C) Compound X has an unsaturation number of 8.
D) Compound X has an unsaturation number of 4.
E) Compound X could contain three double bonds and one triple bond.

Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
22
Complete the reaction by giving the structure of the organic product.
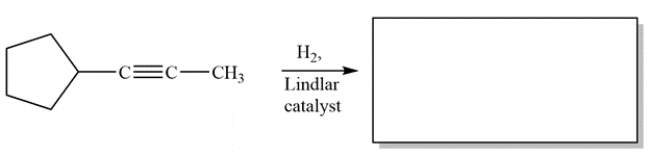


Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
23
Provide an IUPAC name for the following compound.
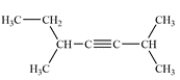


Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
24
A multistep reaction A ⇌ B ⇌ C ⇌ D has the free energy-reaction coordinate diagram shown below. Which step of the reaction is rate-limiting in the forward direction?
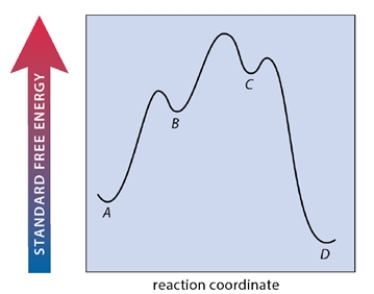
A) the reaction of A to B
B) the reaction of B to C
C) the reaction of C to D

A) the reaction of A to B
B) the reaction of B to C
C) the reaction of C to D

Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
25
This carbocation undergoes rearrangement. Give the structure of the rearranged carbocation and, on the structure given, the curved-arrow notation for the rearrangement.
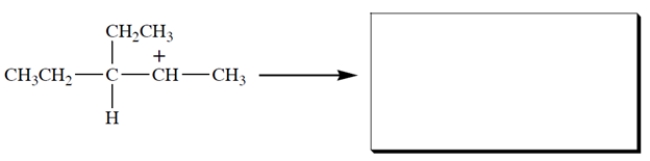


Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
26
Identify the most stable carbocation:
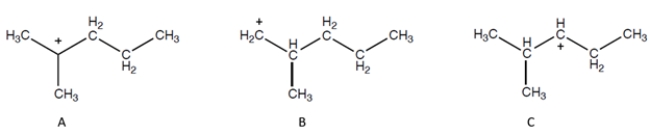
A) carbocation A
B) carbocation B
C) carbocation C

A) carbocation A
B) carbocation B
C) carbocation C

Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck


