Exam 4: Introduction to Alkenes and Alkynes
Exam 1: Chemical Bonding and Chemical Structure26 Questions
Exam 2: Alkanes and Organic Nomenclature25 Questions
Exam 3: The Curved-Arrow Notation, Resonance, Acids and Bases, and Chemical Equilibrium25 Questions
Exam 4: Introduction to Alkenes and Alkynes26 Questions
Exam 5: Addition Reactions of Alkenes and Alkynes25 Questions
Exam 6: Principles of Stereochemistry25 Questions
Exam 7: Cyclic Compounds and Reaction Stereochemistry25 Questions
Exam 8: Nomenclature and Noncovalent Intermolecular Interactions25 Questions
Exam 9: The Chemistry of Alkyl Halides25 Questions
Exam 10: Free-Radical Reactions, Main-Group Organometallic Compounds, and Carbenes25 Questions
Exam 11: The Chemistry of Alcohols and Thiols25 Questions
Exam 12: The Chemistry of Ethers, Epoxides, Glycols, and Sulfides25 Questions
Exam 13: Introduction to Spectroscopy25 Questions
Exam 14: Nuclear Magnetic Resonance Spectroscopy27 Questions
Exam 15: Dienes and Aromaticity25 Questions
Exam 16: The Chemistry of Benzene and Its Derivatives24 Questions
Exam 17: Allylic and Benzylic Reactivity25 Questions
Exam 18: The Chemistry of Aryl Halides, Vinylic Halides, and Phenols25 Questions
Exam 19: The Chemistry of Aldehydes and Ketones25 Questions
Exam 20: The Chemistry of Carboxylic Acids25 Questions
Exam 21: The Chemistry of Carboxylic Acid Derivatives25 Questions
Exam 22: The Chemistry of Enolate Ions, Enols, and Α,β-Unsaturated Carbonyl Compounds25 Questions
Exam 23: The Chemistry of Amines25 Questions
Exam 24: Carbohydrates25 Questions
Exam 25: The Chemistry of Thioesters, Phosphate Esters, and Phosphate Anhydrides25 Questions
Exam 26: The Chemistry of the Aromatic Heterocycles and Nucleic Acids25 Questions
Exam 27: Amino Acids, Peptides, and Proteins25 Questions
Exam 28: Pericyclic Reactions25 Questions
Select questions type
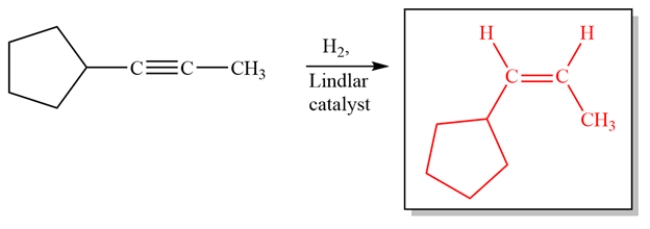
Complete the reaction by giving the structure of the organic product.
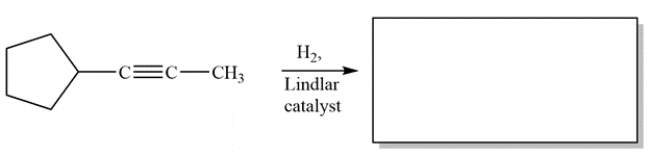
Free
(Essay)
4.9/5  (29)
(29)
Correct Answer:
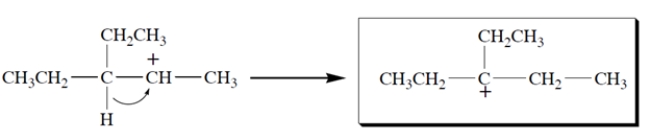
This carbocation undergoes rearrangement. Give the structure of the rearranged carbocation and, on the structure given, the curved-arrow notation for the rearrangement.
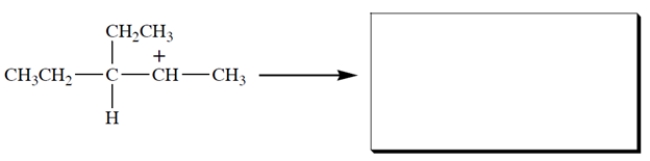
Free
(Essay)
4.9/5  (30)
(30)
Correct Answer:
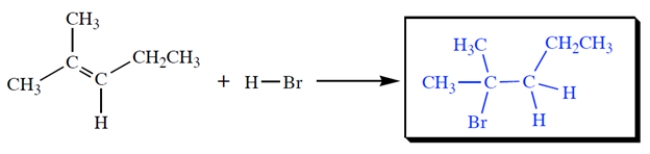
Give the structure of the HBr addition product.
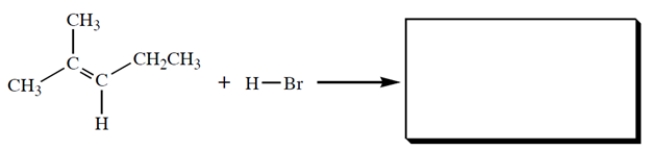
Free
(Essay)
4.9/5  (36)
(36)
Correct Answer:
The Br goes to the more branched carbon and the H to the less branched carbon.
Complete the reaction by giving the products. Show all unshared electron pairs and formal charges.
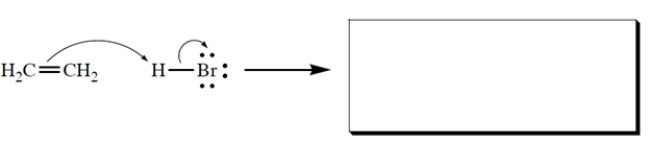
(Essay)
4.8/5  (37)
(37)
Consider this reaction. Draw a curved-arrow mechanism for the reaction. Notice that concentrated acid is present as a catalyst. (You can abbreviate the catalyst as H-A in your mechanism.) This mechanism should be a series of acid-base steps.
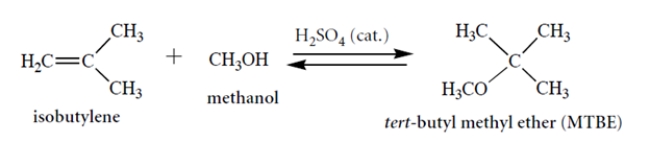
(Essay)
4.9/5  (35)
(35)
Select the true statements that apply to a hydrocarbon, compound X, with the molecular formula C10H14. For purposes of the first two statements, a benzene ring has this skeletal structure:
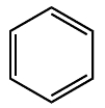
(Multiple Choice)
5.0/5  (37)
(37)
Which of these alkenes has the smallest (or more negative) heat of formation?
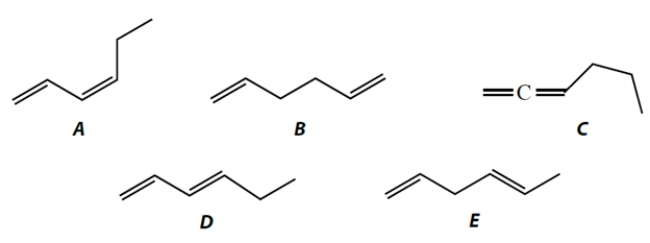
(Multiple Choice)
5.0/5  (36)
(36)
In this pair of compounds, identify the more stable one (as measured by heats of formation).
a.
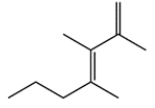 b.
b.
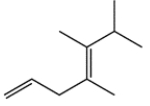
(Multiple Choice)
4.8/5  (37)
(37)
A multistep reaction A ⇌ B ⇌ C ⇌ D has the free energy-reaction coordinate diagram shown below. Which step of the reaction is rate-limiting in the forward direction?
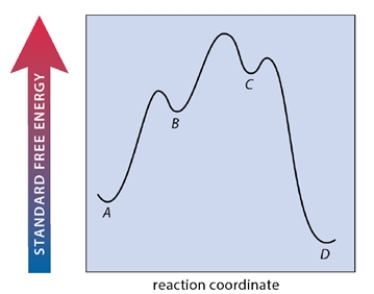
(Multiple Choice)
4.9/5  (27)
(27)
Name this compound (including stereochemical configuration where appropriate).
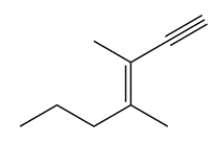
(Short Answer)
4.8/5  (38)
(38)
14. In each pair, identify the more stable one (as measured by heats of formation).
-2:
a.
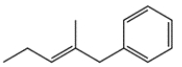 b.
b.
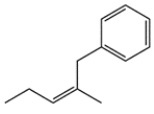 The more stable compound is ________________.
The more stable compound is ________________.
(Multiple Choice)
4.8/5  (28)
(28)
Name the following compound and include the E or Z designation in the name.
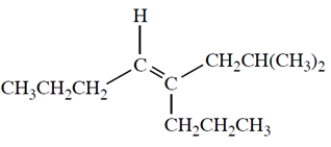
(Short Answer)
4.9/5  (37)
(37)
Consider the reaction free-energy diagram for two reactions that convert reactants (R) into products (P) and select the two correct statements.
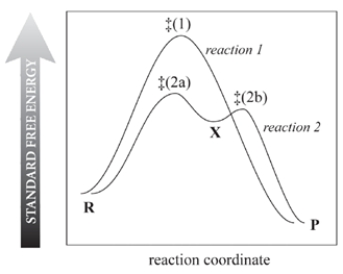
(Multiple Choice)
4.7/5  (27)
(27)
Give the IUPAC name, including stereochemistry, of the compound:
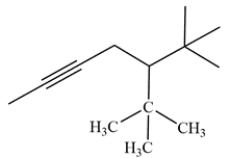
(Short Answer)
4.8/5  (33)
(33)
Showing 1 - 20 of 26
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)