Deck 3: The Curved-Arrow Notation, Resonance, Acids and Bases, and Chemical Equilibrium
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/25
Play
Full screen (f)
Deck 3: The Curved-Arrow Notation, Resonance, Acids and Bases, and Chemical Equilibrium
1
Complete the reaction and draw the curved-arrow notation.
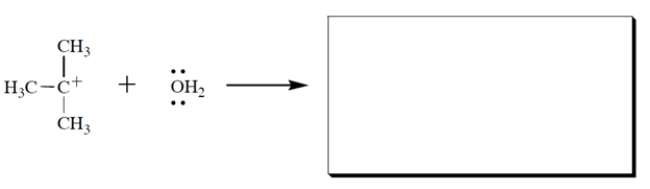

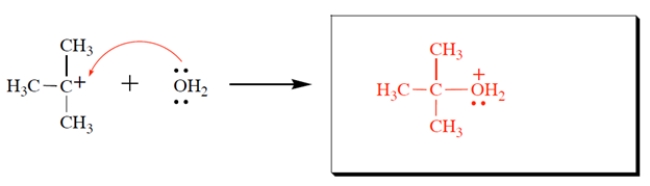
2
Show the curved-arrow notation and the product (including all unshared valence electrons and formal charges, if any) for the Lewis acid-base association reaction of the two species. (Aluminum is in group 3A of the periodic table, beneath boron.) Then identify and label the nucleophile in the reaction.
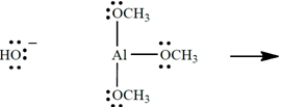

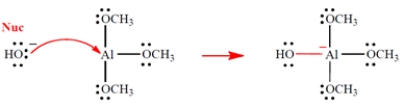
3
Give the products of the electron-pair displacement reaction. (Don't forget unshared pairs and formal charges.) Then circle the nucleophile.
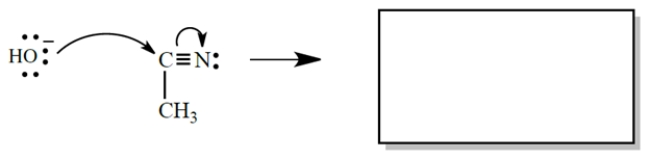

The product of the transformation is:
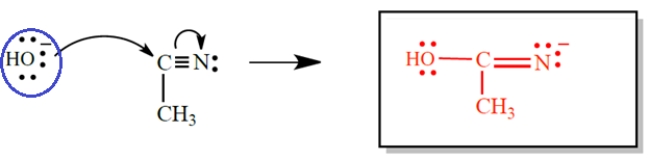 The hydroxide is the nucleophile.
The hydroxide is the nucleophile.
 The hydroxide is the nucleophile.
The hydroxide is the nucleophile. 4
Draw curved arrows and give the products for an electron-pair displacement reaction in which hydroxide ion is the nucleophile and a carbon is the electrophile. Be sure to show all unshared electrons!
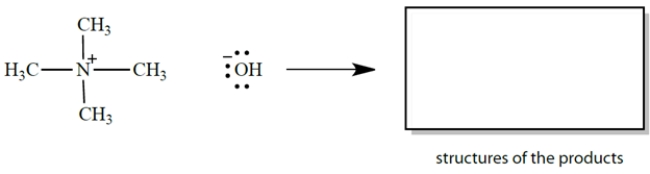


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
5
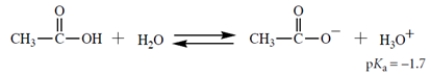
Consider this Brønsted acid-base equilibrium:
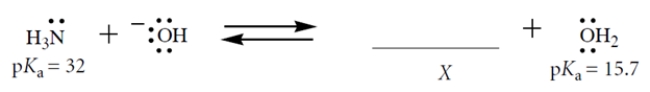 1. Fill in the structure of X; show formal charge and all valence electrons.
1. Fill in the structure of X; show formal charge and all valence electrons.
2. What is Keq for this reaction for the left-to-right direction?
3. If the reaction starts out with 1 M NaOH and 0.5 M NH3 in the solvent water (55.5 M), which species (other than water) is present in the highest concentration at equilibrium?
 1. Fill in the structure of X; show formal charge and all valence electrons.
1. Fill in the structure of X; show formal charge and all valence electrons.2. What is Keq for this reaction for the left-to-right direction?
3. If the reaction starts out with 1 M NaOH and 0.5 M NH3 in the solvent water (55.5 M), which species (other than water) is present in the highest concentration at equilibrium?

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
6
Using curved arrows, show how each resonance structure is converted into the other.



Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
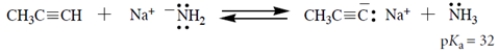
7
Consider the Brønsted acid-base reaction:
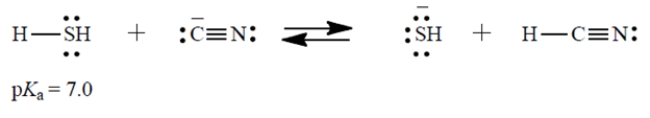
The equilibrium constant for this reaction is 250 (favorable to the right). What is the pKa of HCN (the acid on the right)?

The equilibrium constant for this reaction is 250 (favorable to the right). What is the pKa of HCN (the acid on the right)?

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
8
Determine the standard free-energy change of a reaction with an equilibrium constant of 250, given that 2.3RT at 298 K is 5.7 kJ mol-1. ΔG° = ________________ kJ mol-1.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
9
Complete the electron-pair displacement reaction by showing the curved arrows and the structure(s) of the product(s), including all charges and unshared valence electron pairs. Assume that phosphorus is the nucleophilic atom.
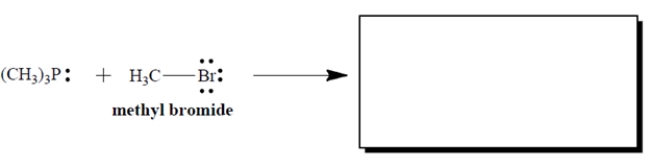


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
10
Aspirin has the structure on the left with a pKa of 3.4.
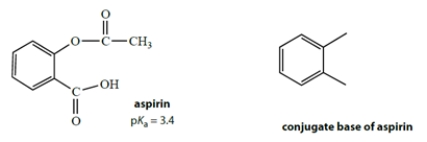 a. Complete the structure on the right for the conjugate base of aspirin.
a. Complete the structure on the right for the conjugate base of aspirin.
b. In a large excess of stomach acid at pH = 2, calculate the fraction of aspirin that is ionized (to 2 significant figures).
 a. Complete the structure on the right for the conjugate base of aspirin.
a. Complete the structure on the right for the conjugate base of aspirin.b. In a large excess of stomach acid at pH = 2, calculate the fraction of aspirin that is ionized (to 2 significant figures).

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
11
In this acid-base equilibrium, the pKa values of the two acids are 4.8 and 9.8, but you must decide which is which from your knowledge of approximate pKa values.
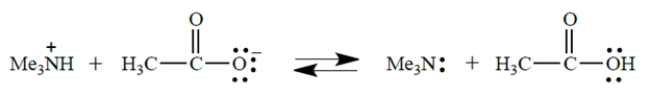 What is the equilibrium constant for this reaction?
What is the equilibrium constant for this reaction?
A) −5
B) +5
C) 10-5
D) 10+5
E)
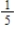
 What is the equilibrium constant for this reaction?
What is the equilibrium constant for this reaction?A) −5
B) +5
C) 10-5
D) 10+5
E)
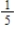

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
12
In the reaction, CH3O− acts as a nucleophile, the phosphorus as an electrophilic center, and CH3S as a leaving group. (Don't be concerned that phosphorus has more than an octet of electrons-that's allowed, and it's irrelevant to the problem.)
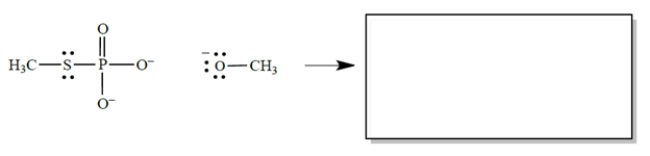 a. In the equation, draw the curved arrows for the process described.
a. In the equation, draw the curved arrows for the process described.
b. In the box, draw the products of the reaction. Don't forget formal charges.
 a. In the equation, draw the curved arrows for the process described.
a. In the equation, draw the curved arrows for the process described.b. In the box, draw the products of the reaction. Don't forget formal charges.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
13
Consider the acid-base equilibrium:
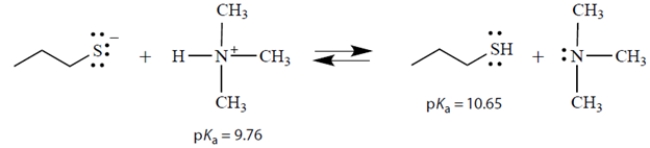 a. What is the equilibrium constant Keq for this reaction?
a. What is the equilibrium constant Keq for this reaction?
b. In the above equation, circle the strongest base.
c. On the left side of the equation, draw the curved arrows that show the formation of the products on the right.
 a. What is the equilibrium constant Keq for this reaction?
a. What is the equilibrium constant Keq for this reaction?b. In the above equation, circle the strongest base.
c. On the left side of the equation, draw the curved arrows that show the formation of the products on the right.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
14
The dissociation reaction for an acid has an associated standard free energy change (ΔG°a) = 28.6 kJ mol−1. What is the pKa of the acid? (2.3RT at 298 K = 5.71 kJ mol−1.)

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
15
Select the strongest acid.
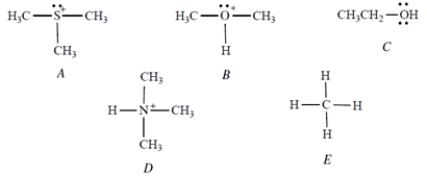
A) Compound A
B) Compound B
C) Compound C
D) Compound D
E) Compound E

A) Compound A
B) Compound B
C) Compound C
D) Compound D
E) Compound E

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
16
Select the correct statement about this reaction:
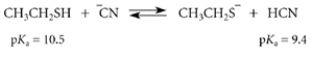
A) The equilibrium lies very far to the right.
B) The equilibrium constant in the left-to-right direction is 1.1.
C) The equilibrium constant in the left-to-right direction is 12.6.
D) The equilibrium constant in the left-to-right direction is 0.08.
E) The equilibrium constant in the left-to-right direction is −1.1.

A) The equilibrium lies very far to the right.
B) The equilibrium constant in the left-to-right direction is 1.1.
C) The equilibrium constant in the left-to-right direction is 12.6.
D) The equilibrium constant in the left-to-right direction is 0.08.
E) The equilibrium constant in the left-to-right direction is −1.1.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
17
Equal amount of compounds A and B (0.01 mol each) are dissolved together in 1 L of water, and this equilibrium is established:
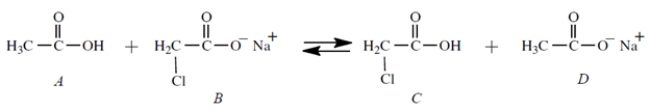 What are the major species in solution at equilibrium? (Select all that apply)
What are the major species in solution at equilibrium? (Select all that apply)
A) Compound A
B) Compound B
C) Compound C
D) Compound D
 What are the major species in solution at equilibrium? (Select all that apply)
What are the major species in solution at equilibrium? (Select all that apply)A) Compound A
B) Compound B
C) Compound C
D) Compound D

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
18
Pivalic acid, which has the structure (CH3)3C-CO2H, has a pKa of 5.2. The standard free energy of ionization of pivalic acid is
________________ kJ mol−1.
________________ kJ mol−1.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
19
Pentothal is a barbiturate used (as its sodium salt) as a short-acting anesthetic. Pentothal is a weak acid with a dissociation constant Ka = 4 × 10−8 M. Which one of the statements best describes the dissociation state of pentothal in the blood, which has a pH of 7.4?
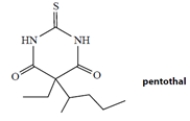
A) more than 90% dissociated
B) between 60% and 90% dissociated
C) about half dissociated
D) between 10% and 40% dissociated
E) less than 10% dissociated

A) more than 90% dissociated
B) between 60% and 90% dissociated
C) about half dissociated
D) between 10% and 40% dissociated
E) less than 10% dissociated

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
20
Choose the number that is closest to the equilibrium constant Keq for this reaction:
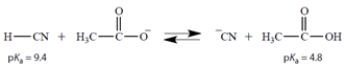
A) 4.6
B) 2.3RT
C) 2.5 × 105
D) 2.5 × 10−5
E) −4.6

A) 4.6
B) 2.3RT
C) 2.5 × 105
D) 2.5 × 10−5
E) −4.6

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
21
Select the equilibrium that lies farthest to the right.
A)
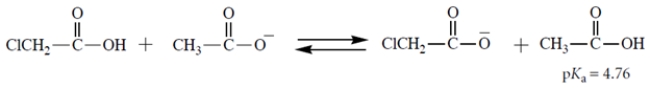
B)
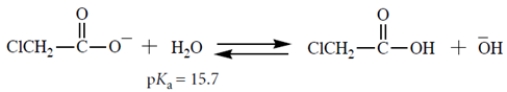
C)
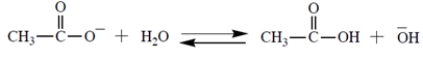
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
22
The free energy of formation (ΔGf°) is defined analogously to a heat of formation: it is the standard free energy for formation of a compound from its elements in their standard states at 298 K. Consider the following reaction and their associated standard free energies of formation (all in kJ mol−1). Although these values are for the gas phase, they give us some idea of what to expect in solution.
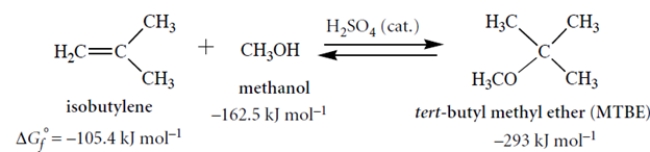 MTBE has been an important gasoline additive until it began to be replaced by ethanol in 2002.
MTBE has been an important gasoline additive until it began to be replaced by ethanol in 2002.
Calculate the equilibrium constant for this reaction in the left-to-right direction. Show your work.
 MTBE has been an important gasoline additive until it began to be replaced by ethanol in 2002.
MTBE has been an important gasoline additive until it began to be replaced by ethanol in 2002.Calculate the equilibrium constant for this reaction in the left-to-right direction. Show your work.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
23
Select the equilibrium that most favors products at equilibrium.
A)

B)

C)

D)

E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
24
Predict the approximate equilibrium constant (as a power of 10) for the reaction.



Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
25
Ascorbic acid (Vitamin C) is a diprotic acid with two pKa values; pKa1 = 4.2 and pKa2 = 11.6. The two acidic groups are the two -OH groups, indicated by arrows in the structure. If we let AH2 represent ascorbic acid, its successive ionized species can be abbreviated by −AH and A2−.
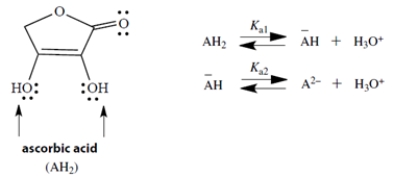 Draw the structure of −AH and its two resonance structures. Draw the curved arrow notation to convert each structure to the next. (Hint: In one structure, charge is delocalized into the C=O group.)
Draw the structure of −AH and its two resonance structures. Draw the curved arrow notation to convert each structure to the next. (Hint: In one structure, charge is delocalized into the C=O group.)
 Draw the structure of −AH and its two resonance structures. Draw the curved arrow notation to convert each structure to the next. (Hint: In one structure, charge is delocalized into the C=O group.)
Draw the structure of −AH and its two resonance structures. Draw the curved arrow notation to convert each structure to the next. (Hint: In one structure, charge is delocalized into the C=O group.)
Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck


