Exam 3: The Curved-Arrow Notation, Resonance, Acids and Bases, and Chemical Equilibrium
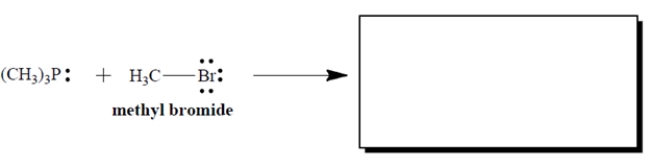
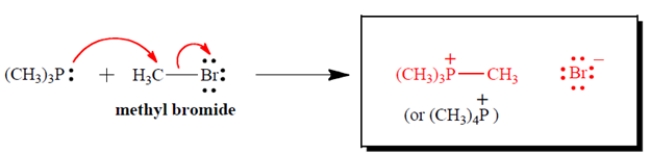
Complete the electron-pair displacement reaction by showing the curved arrows and the structure(s) of the product(s), including all charges and unshared valence electron pairs. Assume that phosphorus is the nucleophilic atom.
The free energy of formation (ΔGf°) is defined analogously to a heat of formation: it is the standard free energy for formation of a compound from its elements in their standard states at 298 K. Consider the following reaction and their associated standard free energies of formation (all in kJ mol−1). Although these values are for the gas phase, they give us some idea of what to expect in solution.
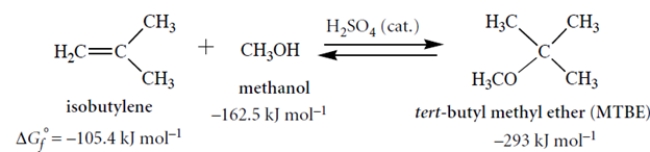 MTBE has been an important gasoline additive until it began to be replaced by ethanol in 2002.
Calculate the equilibrium constant for this reaction in the left-to-right direction. Show your work.
MTBE has been an important gasoline additive until it began to be replaced by ethanol in 2002.
Calculate the equilibrium constant for this reaction in the left-to-right direction. Show your work.
The ΔG° for the reaction is obtained by subtracting the ΔGf° of the reactants from the ΔGf° of the products. Thus,
ΔG° (reaction) = −293 − (−162.5 − 105.4) = −25.1 kJ mol−1
ΔG° = −2.3RTlog Keq
log Keq = −ΔG°/2.3RT = −(−25.1)/5.7 = 4.4
Keq = 104.4 or 2.53 × 104 atm
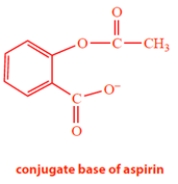
Aspirin has the structure on the left with a pKa of 3.4.
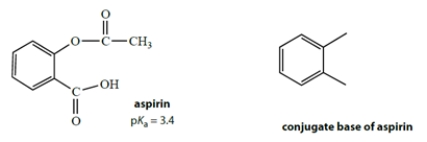 a. Complete the structure on the right for the conjugate base of aspirin.
b. In a large excess of stomach acid at pH = 2, calculate the fraction of aspirin that is ionized (to 2 significant figures).
a. Complete the structure on the right for the conjugate base of aspirin.
b. In a large excess of stomach acid at pH = 2, calculate the fraction of aspirin that is ionized (to 2 significant figures).
a. The conjugate base of aspirin is formed by losing the acidic proton. The pKa tells us that this is the carboxylic acid is the acidic group. The conjugate acid is the following anion:
 b. Calculate the fraction dissociated from the Henderson-Hasselbalch equation or, more easily, from Eq. 3.67a, with Δ = pH − pKa = 2 − 3.4 = −1.4.
b. Calculate the fraction dissociated from the Henderson-Hasselbalch equation or, more easily, from Eq. 3.67a, with Δ = pH − pKa = 2 − 3.4 = −1.4.
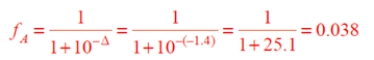 Therefore, aspirin is 3.8% ionized under these conditions. (If there is any doubt whether to use the equation with the Δ or −Δ in the denominator, we know that the fraction ionization is small because the pH is much lower than the pKa. Therefore, the second term in the denominator must be large.)
Therefore, aspirin is 3.8% ionized under these conditions. (If there is any doubt whether to use the equation with the Δ or −Δ in the denominator, we know that the fraction ionization is small because the pH is much lower than the pKa. Therefore, the second term in the denominator must be large.)
Give the products of the electron-pair displacement reaction. (Don't forget unshared pairs and formal charges.) Then circle the nucleophile.
Using curved arrows, show how each resonance structure is converted into the other.

Consider the acid-base equilibrium:
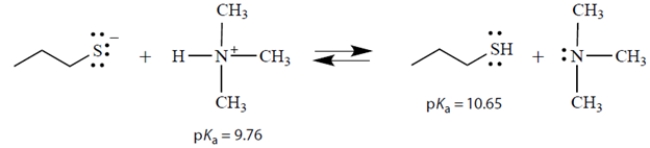 a. What is the equilibrium constant Keq for this reaction?
b. In the above equation, circle the strongest base.
c. On the left side of the equation, draw the curved arrows that show the formation of the products on the right.
a. What is the equilibrium constant Keq for this reaction?
b. In the above equation, circle the strongest base.
c. On the left side of the equation, draw the curved arrows that show the formation of the products on the right.
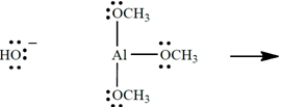
Show the curved-arrow notation and the product (including all unshared valence electrons and formal charges, if any) for the Lewis acid-base association reaction of the two species. (Aluminum is in group 3A of the periodic table, beneath boron.) Then identify and label the nucleophile in the reaction.
Select the equilibrium that most favors products at equilibrium.
Choose the number that is closest to the equilibrium constant Keq for this reaction:
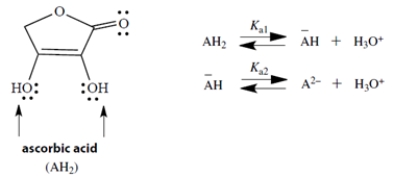
Ascorbic acid (Vitamin C) is a diprotic acid with two pKa values; pKa1 = 4.2 and pKa2 = 11.6. The two acidic groups are the two -OH groups, indicated by arrows in the structure. If we let AH2 represent ascorbic acid, its successive ionized species can be abbreviated by −AH and A2−.
 Draw the structure of −AH and its two resonance structures. Draw the curved arrow notation to convert each structure to the next. (Hint: In one structure, charge is delocalized into the C=O group.)
Draw the structure of −AH and its two resonance structures. Draw the curved arrow notation to convert each structure to the next. (Hint: In one structure, charge is delocalized into the C=O group.)
The dissociation reaction for an acid has an associated standard free energy change (ΔG°a) = 28.6 kJ mol−1. What is the pKa of the acid? (2.3RT at 298 K = 5.71 kJ mol−1.)
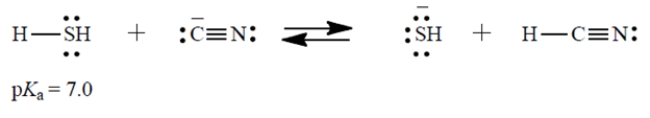
Consider the Brønsted acid-base reaction:
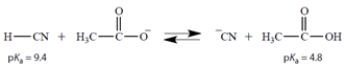
The equilibrium constant for this reaction is 250 (favorable to the right). What is the pKa of HCN (the acid on the right)?
The equilibrium constant for this reaction is 250 (favorable to the right). What is the pKa of HCN (the acid on the right)?
In this acid-base equilibrium, the pKa values of the two acids are 4.8 and 9.8, but you must decide which is which from your knowledge of approximate pKa values.
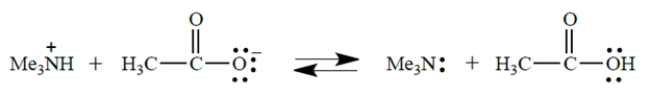 What is the equilibrium constant for this reaction?
What is the equilibrium constant for this reaction?
Select the correct statement about this reaction:
+\rightleftarrows+ =10.5 p=9.4
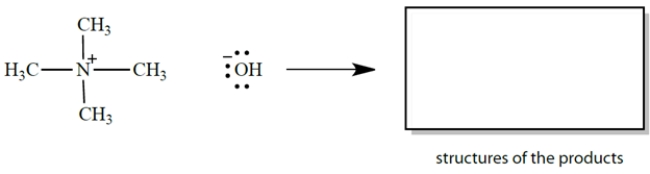
Draw curved arrows and give the products for an electron-pair displacement reaction in which hydroxide ion is the nucleophile and a carbon is the electrophile. Be sure to show all unshared electrons!
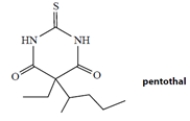
Pentothal is a barbiturate used (as its sodium salt) as a short-acting anesthetic. Pentothal is a weak acid with a dissociation constant Ka = 4 × 10−8 M. Which one of the statements best describes the dissociation state of pentothal in the blood, which has a pH of 7.4?
Pivalic acid, which has the structure (CH3)3C-CO2H, has a pKa of 5.2. The standard free energy of ionization of pivalic acid is
________________ kJ mol−1.
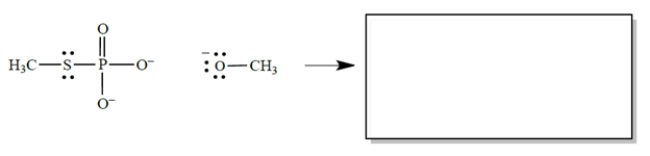
In the reaction, CH3O− acts as a nucleophile, the phosphorus as an electrophilic center, and CH3S as a leaving group. (Don't be concerned that phosphorus has more than an octet of electrons-that's allowed, and it's irrelevant to the problem.)
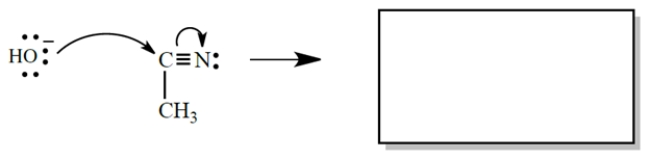
 a. In the equation, draw the curved arrows for the process described.
b. In the box, draw the products of the reaction. Don't forget formal charges.
a. In the equation, draw the curved arrows for the process described.
b. In the box, draw the products of the reaction. Don't forget formal charges.
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)