Deck 13: Introduction to Spectroscopy
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/25
Play
Full screen (f)
Deck 13: Introduction to Spectroscopy
1
What is the energy (in kJ mol-1) of infrared radiation that has a wavenumber of 1600 cm-1? (Planck's constant = 3.99 × 10-13 kJ mol-1 sec; the velocity of light = 3 × 1010 cm sec-1)
Even if you do not remember the formula, this sort of problem can be done by dimensional analysis:
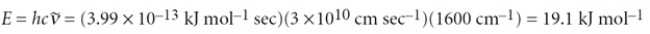
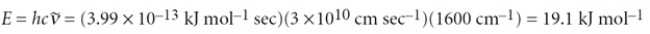
2
Typical IR absorptions occur in the 650-3700 cm-1 wavenumber range. These result from energy absorption from IR radiation by
(Pay attention to your units!)
A) molecular vibrations that occur roughly 1013 to 1014 times per second.
B) molecular rotations that occur roughly 1013 to 1014 times per second.
C) molecular vibrations that occur roughly 10-13 to 10-14 times per second.
D) molecular vibrations that occur roughly 1010-1011 times per second
E) molecular vibrations that occur roughly 10-11 to 10-12 times per second.
(Pay attention to your units!)
A) molecular vibrations that occur roughly 1013 to 1014 times per second.
B) molecular rotations that occur roughly 1013 to 1014 times per second.
C) molecular vibrations that occur roughly 10-13 to 10-14 times per second.
D) molecular vibrations that occur roughly 1010-1011 times per second
E) molecular vibrations that occur roughly 10-11 to 10-12 times per second.
A
3
The -C≡N stretching frequency in the infrared spectrum occurs at about 2220 cm-1. The C-N stretching frequency should occur at about
A) 740 cm-1
B) 1010 cm-1
C) 1280 cm-1
D) 1820 cm-1
A) 740 cm-1
B) 1010 cm-1
C) 1280 cm-1
D) 1820 cm-1
A
4
Select the two true statements.
A) An organic compound with a dipole moment absorbs all wavelengths of IR radiation equally.
B) Wavelength is proportional to the energy of a light wave.
C) IR absorption is caused by the interaction of the electric field of light with a vibrating bond dipole.
D) A necessary but not sufficient condition for IR absorption is a match between the frequency of the vibrational mode and the frequency of light.
E) A sufficient condition for IR absorption is a match between the frequency of the vibrational mode and the frequency of light.f.
Every IR absorption corresponds to the vibration of an individual bond.
A) An organic compound with a dipole moment absorbs all wavelengths of IR radiation equally.
B) Wavelength is proportional to the energy of a light wave.
C) IR absorption is caused by the interaction of the electric field of light with a vibrating bond dipole.
D) A necessary but not sufficient condition for IR absorption is a match between the frequency of the vibrational mode and the frequency of light.
E) A sufficient condition for IR absorption is a match between the frequency of the vibrational mode and the frequency of light.f.
Every IR absorption corresponds to the vibration of an individual bond.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
5
Select the true statement.
A) The S-H stretching absorption in the IR should occur at higher wavenumber than the C-H stretching absorption primarily because S has a greater mass than C.
B) The S-H stretching absorption in the IR should occur at higher wavenumber than the C-H stretching absorption primarily because the S-H bond is weaker than the C-H bond.
C) The S-H stretching absorption in the IR should occur at lower wavenumber than the C-H stretching absorption primarily because the S-H bond is stronger than the C-H bond.
D) The S-H stretching absorption in the IR should occur at lower wavenumber than the C-H stretching absorption primarily because the S-H bond is weaker than the C-H bond.
E) The S-H stretching absorption in the IR should occur at lower wavenumber than the C-H stretching absorption primarily because S has a greater mass than C.
A) The S-H stretching absorption in the IR should occur at higher wavenumber than the C-H stretching absorption primarily because S has a greater mass than C.
B) The S-H stretching absorption in the IR should occur at higher wavenumber than the C-H stretching absorption primarily because the S-H bond is weaker than the C-H bond.
C) The S-H stretching absorption in the IR should occur at lower wavenumber than the C-H stretching absorption primarily because the S-H bond is stronger than the C-H bond.
D) The S-H stretching absorption in the IR should occur at lower wavenumber than the C-H stretching absorption primarily because the S-H bond is weaker than the C-H bond.
E) The S-H stretching absorption in the IR should occur at lower wavenumber than the C-H stretching absorption primarily because S has a greater mass than C.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
6
In IR spectroscopy, an absorption occurs any time
A) a vibration is located in a magnetic field.
B) vibrating bonds are conjugated.
C) any vibrating bond interacts with a light wave vibrating with the same frequency.
D) a vibrating dipole moment interacts with a light wave vibrating with the same frequency.
E) the mass of one atom involved in the vibration is much less than the mass of the others.
A) a vibration is located in a magnetic field.
B) vibrating bonds are conjugated.
C) any vibrating bond interacts with a light wave vibrating with the same frequency.
D) a vibrating dipole moment interacts with a light wave vibrating with the same frequency.
E) the mass of one atom involved in the vibration is much less than the mass of the others.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
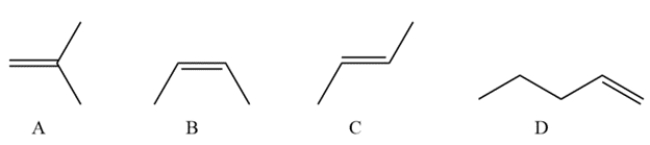
7
Which of the compounds would have the strongest C=C stretching absorption in its IR spectrum?
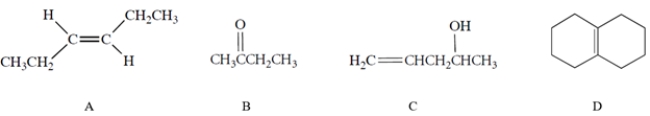
A) compound A
B) compound B
C) compound C
D) compound D
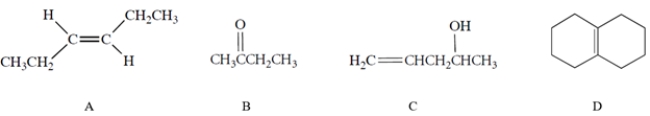
A) compound A
B) compound B
C) compound C
D) compound D

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
8
The C=C stretching absorption in the IR spectrum occurs at about 1650 cm-1. The C-C stretching vibration occurs but is not observed. Which of these frequencies would be closest to the frequency of the C-C stretching vibration?
A) 3300 cm-1
B) 815 cm-1
C) 2330 cm-1
D) 1165 cm-1
E) (1650 cm-1)/2c, where c is the speed of light.
A) 3300 cm-1
B) 815 cm-1
C) 2330 cm-1
D) 1165 cm-1
E) (1650 cm-1)/2c, where c is the speed of light.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
9
When the epoxy thiol is treated with dilute acid in ethanol solution, an isomer is formed that shows a broad stretch at 3400 cm-1 in its IR spectrum. Using mechanistic reasoning with the curved-arrow notation, predict the structure of the product and its stereochemistry.
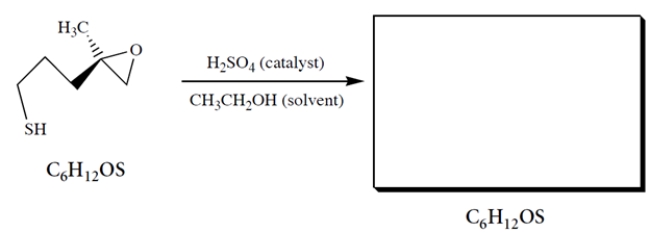


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
10
Which molecular vibration does not have an absorption in the IR spectrum?
A) O=C=O symmetrical stretch
B) O=C=O unsymmetrical stretch
C) the carbonyl stretch in acetone, CH3C(=O)CH3
D) the alkene stretch in CH2=C(CH3)2
A) O=C=O symmetrical stretch
B) O=C=O unsymmetrical stretch
C) the carbonyl stretch in acetone, CH3C(=O)CH3
D) the alkene stretch in CH2=C(CH3)2

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
11
The IR absorption that occurs at the highest wavenumber is
A) the C≡C stretching vibration of 1-butyne.
B) the C=C stretching vibration of 1-butene.
C) the asymmetric C-O stretching vibration of diethyl ether.
D) the =C-H bending vibrations of 1-butene.
A) the C≡C stretching vibration of 1-butyne.
B) the C=C stretching vibration of 1-butene.
C) the asymmetric C-O stretching vibration of diethyl ether.
D) the =C-H bending vibrations of 1-butene.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
12
Identify the compound that corresponds to the IR spectrum.
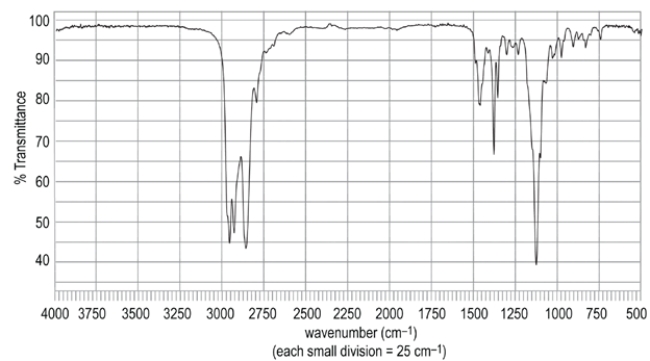
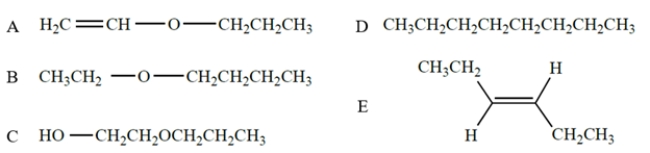
A) compound A
B) compound B
C) compound C
D) compound D
E) compound E

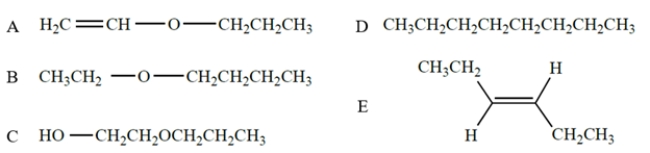
A) compound A
B) compound B
C) compound C
D) compound D
E) compound E

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
13
Use the structure, including the labels on appropriate protons and bonds, to answer the questions. Please note: Protons are uppercase, bonds are lowercase.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
14
Which compound has an absorption at 700 cm-1?
A) compound A
B) compound B
C) compound C
D) compound D

A) compound A
B) compound B
C) compound C
D) compound D

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
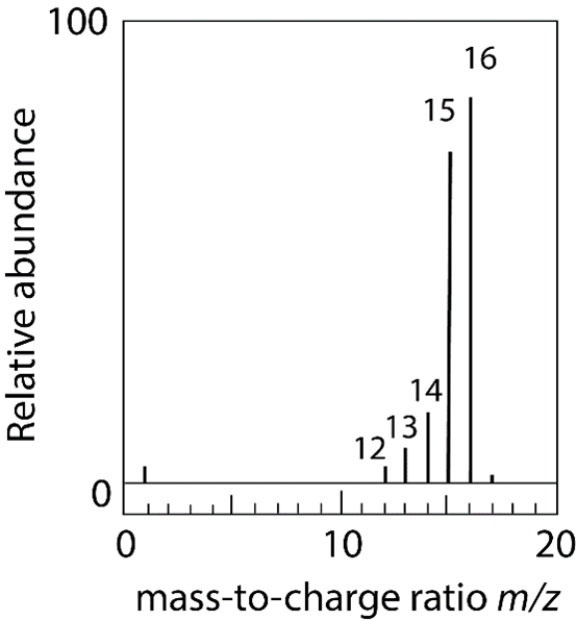
15
Methane (CH4) is vaporized in a vacuum chamber and bombarded with an electron beam of high energy to give the mass spectrum below. (a) Identify each of the labeled m/z peaks. (b) Explain the presence of the small peak at m/z 17.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
16
Which species shown would not be detected in the electron ionization (EI) mass spectrometry?
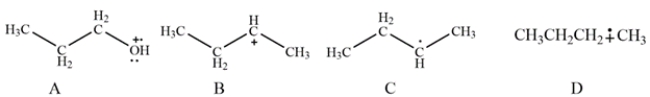
A) species A
B) species B
C) species C
D) species D

A) species A
B) species B
C) species C
D) species D

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
17
The base peak of 2,2-dimethylpentane is at m/z 57, which corresponds to a composition of C4H9.
(a) Suggest a structure for the fragment that accounts for this peak.
(b) Offer a reason that this fragment is so abundant.
(c) Give a mechanism that shows the formation of this fragment.
(a) Suggest a structure for the fragment that accounts for this peak.
(b) Offer a reason that this fragment is so abundant.
(c) Give a mechanism that shows the formation of this fragment.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
18
The electron ionization mass spectrum of 1-hexanol barely exhibits a molecular ion peak at m/z 102 due to rapid fragmentation to the next largest fragment at m/z 84. Predict the structure corresponding to the fragment and draw a curved arrow mechanism to show how it is formed.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
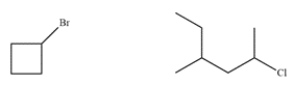
19
These two compounds have similar molecular weights. Explain how to use mass spectrometry to differentiate between the two.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
20
A compound with an unknown structure is investigated by determining both its EI (electron ionization) and CI (chemical ionization) mass spectra. Why would you use both and what information is gleaned from each?

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
21
The electron ionization of an unknown neutral compound gives this mass spectrum. Which combination of elements most likely comprises the molecule?
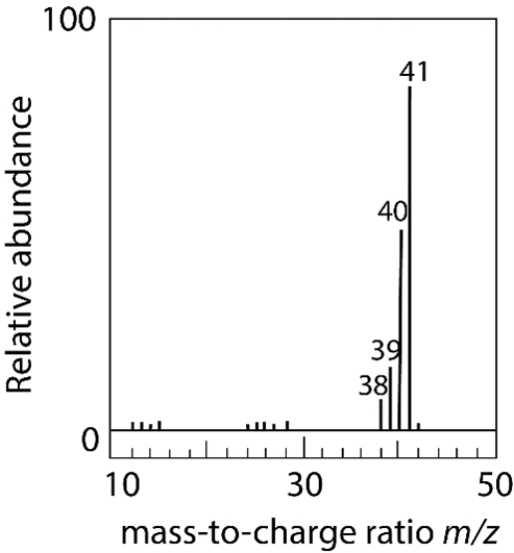
A) two carbons, three hydrogens, and one nitrogen
B) three carbons and five hydrogens
C) one carbon, five hydrogens, and two nitrogen atoms
D) two carbons, four hydrogens, and one nitrogen

A) two carbons, three hydrogens, and one nitrogen
B) three carbons and five hydrogens
C) one carbon, five hydrogens, and two nitrogen atoms
D) two carbons, four hydrogens, and one nitrogen

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
22
Menthol is obtained from oils of peppermint and spearmint and is often added to cough drops to give a minty, cooling sensation. In the electron ionization mass spectrum of menthol, the molecular ion peak is not observed, but the largest fragment has an m/z 138 at 27% relative abundance and an ion at m/z 139 with a 3% relative abundance. Given the two possible compounds, identify which is menthol and explain how you arrived at that conclusion.
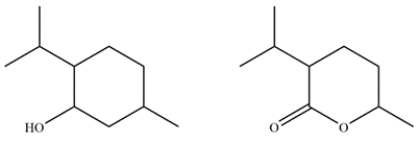


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
23
Two students perform a hydrolysis reaction on cis-2-pentene. Two alcohols are isolated and one of the students argues that he can prove the structure of each using electron ionization mass spectrometry. Do you agree with his argument? Explain.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
24
A graduate student is cleaning out the refrigerator in the laboratory and stumbles upon an unlabeled beaker in the back. The IR analysis of the compound shows a large broad peak at 3300 cm-1 and no other significant peaks. The electron ionization mass spectrum shows the molecular ion at m/z 100 at 15% relative abundance and an ion at m/z 101 with a 1% relative abundance. Propose a structure for the unknown compound. Hint: Start with the relative abundance data to determine the number of carbons in the molecule.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
25
Outline a synthesis for the transformation. Describe how you could use IR spectroscopy or mass spectrometry to monitor the completion of the reaction.
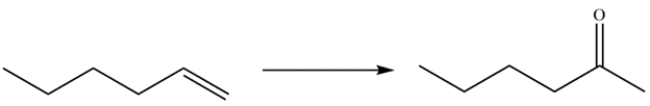


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck


