Exam 13: Introduction to Spectroscopy
Exam 1: Chemical Bonding and Chemical Structure26 Questions
Exam 2: Alkanes and Organic Nomenclature25 Questions
Exam 3: The Curved-Arrow Notation, Resonance, Acids and Bases, and Chemical Equilibrium25 Questions
Exam 4: Introduction to Alkenes and Alkynes26 Questions
Exam 5: Addition Reactions of Alkenes and Alkynes25 Questions
Exam 6: Principles of Stereochemistry25 Questions
Exam 7: Cyclic Compounds and Reaction Stereochemistry25 Questions
Exam 8: Nomenclature and Noncovalent Intermolecular Interactions25 Questions
Exam 9: The Chemistry of Alkyl Halides25 Questions
Exam 10: Free-Radical Reactions, Main-Group Organometallic Compounds, and Carbenes25 Questions
Exam 11: The Chemistry of Alcohols and Thiols25 Questions
Exam 12: The Chemistry of Ethers, Epoxides, Glycols, and Sulfides25 Questions
Exam 13: Introduction to Spectroscopy25 Questions
Exam 14: Nuclear Magnetic Resonance Spectroscopy27 Questions
Exam 15: Dienes and Aromaticity25 Questions
Exam 16: The Chemistry of Benzene and Its Derivatives24 Questions
Exam 17: Allylic and Benzylic Reactivity25 Questions
Exam 18: The Chemistry of Aryl Halides, Vinylic Halides, and Phenols25 Questions
Exam 19: The Chemistry of Aldehydes and Ketones25 Questions
Exam 20: The Chemistry of Carboxylic Acids25 Questions
Exam 21: The Chemistry of Carboxylic Acid Derivatives25 Questions
Exam 22: The Chemistry of Enolate Ions, Enols, and Α,β-Unsaturated Carbonyl Compounds25 Questions
Exam 23: The Chemistry of Amines25 Questions
Exam 24: Carbohydrates25 Questions
Exam 25: The Chemistry of Thioesters, Phosphate Esters, and Phosphate Anhydrides25 Questions
Exam 26: The Chemistry of the Aromatic Heterocycles and Nucleic Acids25 Questions
Exam 27: Amino Acids, Peptides, and Proteins25 Questions
Exam 28: Pericyclic Reactions25 Questions
Select questions type
In IR spectroscopy, an absorption occurs any time
Free
(Multiple Choice)
4.7/5  (32)
(32)
Correct Answer:
D
What is the energy (in kJ mol-1) of infrared radiation that has a wavenumber of 1600 cm-1? (Planck's constant = 3.99 × 10-13 kJ mol-1 sec; the velocity of light = 3 × 1010 cm sec-1)
Free
(Essay)
4.9/5  (37)
(37)
Correct Answer:
Even if you do not remember the formula, this sort of problem can be done by dimensional analysis:
A compound with an unknown structure is investigated by determining both its EI (electron ionization) and CI (chemical ionization) mass spectra. Why would you use both and what information is gleaned from each?
Free
(Essay)
4.9/5  (34)
(34)
Correct Answer:
Molecular ions in electron ionization are very high energy and likely to undergo fragmentation. In many cases, the molecular ion is not visible in the spectrum or too weak to be diagnostic. Chemical ionization is a milder process, where a gas is ionized instead, leading to formation of positive ions with the unknown structure. This leads to an easily identifiable M+1 peak, allowing the user to deduce the molecular mass. Electron ionization gives complementary fragmentation information, which can be used to deduce other aspects of the structure.
Which species shown would not be detected in the electron ionization (EI) mass spectrometry?
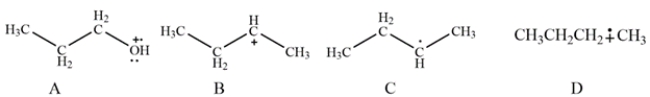
(Multiple Choice)
4.7/5  (44)
(44)
The C=C stretching absorption in the IR spectrum occurs at about 1650 cm-1. The C-C stretching vibration occurs but is not observed. Which of these frequencies would be closest to the frequency of the C-C stretching vibration?
(Multiple Choice)
4.7/5  (40)
(40)
The -C≡N stretching frequency in the infrared spectrum occurs at about 2220 cm-1. The C-N stretching frequency should occur at about
(Multiple Choice)
4.9/5  (33)
(33)
Methane (CH4) is vaporized in a vacuum chamber and bombarded with an electron beam of high energy to give the mass spectrum below. (a) Identify each of the labeled m/z peaks. (b) Explain the presence of the small peak at m/z 17.
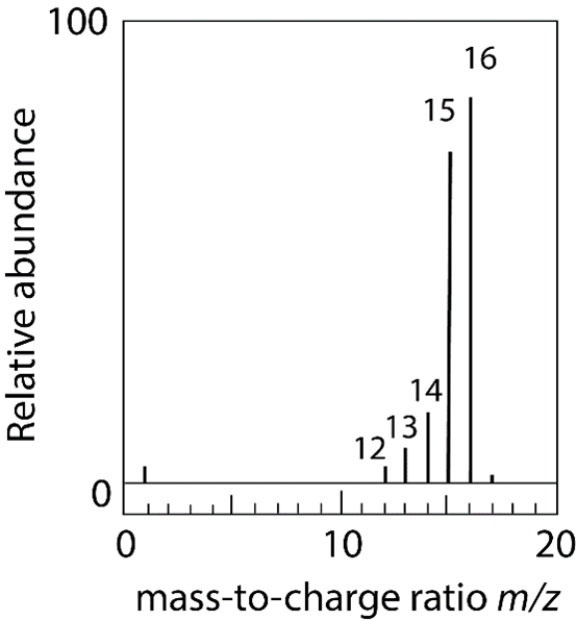
(Essay)
4.8/5  (35)
(35)
Two students perform a hydrolysis reaction on cis-2-pentene. Two alcohols are isolated and one of the students argues that he can prove the structure of each using electron ionization mass spectrometry. Do you agree with his argument? Explain.
(Essay)
4.9/5  (35)
(35)
Use the structure, including the labels on appropriate protons and bonds, to answer the questions. Please note: Protons are uppercase, bonds are lowercase.
(Short Answer)
4.9/5  (33)
(33)
Which molecular vibration does not have an absorption in the IR spectrum?
(Multiple Choice)
4.8/5  (31)
(31)
Outline a synthesis for the transformation. Describe how you could use IR spectroscopy or mass spectrometry to monitor the completion of the reaction.
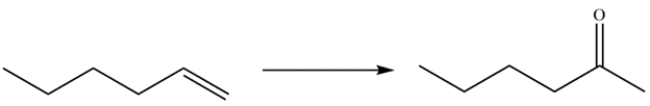
(Essay)
4.8/5  (38)
(38)
The electron ionization mass spectrum of 1-hexanol barely exhibits a molecular ion peak at m/z 102 due to rapid fragmentation to the next largest fragment at m/z 84. Predict the structure corresponding to the fragment and draw a curved arrow mechanism to show how it is formed.
(Essay)
4.9/5  (37)
(37)
These two compounds have similar molecular weights. Explain how to use mass spectrometry to differentiate between the two.
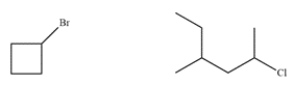
(Essay)
4.9/5  (45)
(45)
The electron ionization of an unknown neutral compound gives this mass spectrum. Which combination of elements most likely comprises the molecule?
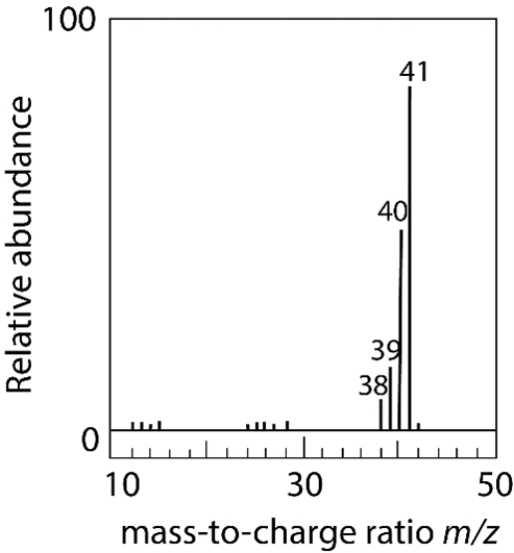
(Multiple Choice)
4.9/5  (42)
(42)
A graduate student is cleaning out the refrigerator in the laboratory and stumbles upon an unlabeled beaker in the back. The IR analysis of the compound shows a large broad peak at 3300 cm-1 and no other significant peaks. The electron ionization mass spectrum shows the molecular ion at m/z 100 at 15% relative abundance and an ion at m/z 101 with a 1% relative abundance. Propose a structure for the unknown compound. Hint: Start with the relative abundance data to determine the number of carbons in the molecule.
(Essay)
4.9/5  (35)
(35)
Typical IR absorptions occur in the 650-3700 cm-1 wavenumber range. These result from energy absorption from IR radiation by
(Pay attention to your units!)
(Multiple Choice)
4.8/5  (28)
(28)
Showing 1 - 20 of 25
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)