Deck 21: Thermodynamics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/50
Play
Full screen (f)
Deck 21: Thermodynamics
1
The perpetual motion machine of the first kind is
A) a device that extracts thermal energy from a heat source and converts it into mechanical energy.
B) a device that supplies endless output of work without input of fuel.
C) a Carnot engine.
D) none of the above.
A) a device that extracts thermal energy from a heat source and converts it into mechanical energy.
B) a device that supplies endless output of work without input of fuel.
C) a Carnot engine.
D) none of the above.
a device that supplies endless output of work without input of fuel.
2
The perpetual motion machine of the second kind is
A) a device that extracts thermal energy from a heat source and converts it into mechanical energy.
B) a device that supplies endless output of work without input of fuel.
C) a Carnot engine.
D) none of the above.
A) a device that extracts thermal energy from a heat source and converts it into mechanical energy.
B) a device that supplies endless output of work without input of fuel.
C) a Carnot engine.
D) none of the above.
a device that extracts thermal energy from a heat source and converts it into mechanical energy.
3
The metric unit associated with the amount of heat transferred, Q, is
A) watt.
B) Btu.
C) joule.
D) newton.
A) watt.
B) Btu.
C) joule.
D) newton.
joule.
4
The metric unit associated with entropy is
A) joule/kelvin.
B) joule.
C) joule·kelvin.
D) kelvin/joule.
A) joule/kelvin.
B) joule.
C) joule·kelvin.
D) kelvin/joule.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
5
During an adiabatic process
A) Q is greater than zero.
B) Q is less than zero.
C) Q is equal to zero.
D) all of the above are possible.
A) Q is greater than zero.
B) Q is less than zero.
C) Q is equal to zero.
D) all of the above are possible.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
6
During an adiabatic process the work done by the system is
A) greater than zero.
B) less than zero.
C) equal to zero.
D) any of the above.
A) greater than zero.
B) less than zero.
C) equal to zero.
D) any of the above.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
7
During an adiabatic process the change in the temperature of the system is
A) greater than zero.
B) less than zero.
C) equal to zero.
D) any of the above.
A) greater than zero.
B) less than zero.
C) equal to zero.
D) any of the above.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
8
During an expanding process 140 J of heat is added to a system and 150 J of work is done by the system. The change in the internal energy of the system is
A) 10 J.
B) -10 J.
C) 150 J.
D) -290 J.
A) 10 J.
B) -10 J.
C) 150 J.
D) -290 J.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
9
During an expanding process 140 J of heat is removed from a system and 150 J of work is done by the system. The change in the internal energy of the system is
A) 10 J.
B) -10 J.
C) 150 J.
D) -290 J.
A) 10 J.
B) -10 J.
C) 150 J.
D) -290 J.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
10
A gas at a temperature T1 undergoes an isothermal free expansion. The First Law of Thermodynamics tells us that the change in the internal energy of the gas
A) increases.
B) decreases.
C) depends on the temperature T1.
D) None of the above answers is correct.
A) increases.
B) decreases.
C) depends on the temperature T1.
D) None of the above answers is correct.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
11
A heat engine produces a power output of 1.5 * 105 W when it receives 50.0 MJ of heat per minute. The efficiency of the engine is
A) 0.18.
B) 0.30.
C) 0.03.
D) 0.003.
A) 0.18.
B) 0.30.
C) 0.03.
D) 0.003.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
12
The efficiency of a heat engine is 0.20 when it is expelling 4.0 108 J of heat. The work that the engine is producing is
A) 1.0 108 J.
B) 5.0 108 J.
C) 8.0 107 J.
D) 2.0 108 J.
A) 1.0 108 J.
B) 5.0 108 J.
C) 8.0 107 J.
D) 2.0 108 J.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
13
One-quarter of the energy a heat engine receives is expelled as waste. The theoretical efficiency of this system
A) is 0.25.
B) is 0.50.
C) is 0.75.
D) cannot be determined from the information given.
A) is 0.25.
B) is 0.50.
C) is 0.75.
D) cannot be determined from the information given.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
14
The graph here represents a p-V diagram where the x axis is the volume in units of 10-3 m3 and the y axis is the pressure in units of 105 N/m2. The work done by the gas as it expands from (p1, V1) to (p2, V2) along the path indicated is
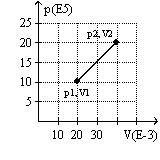
A) 30 kJ.
B) 10 kJ
C) -30 kJ.
D) 10 kJ.

A) 30 kJ.
B) 10 kJ
C) -30 kJ.
D) 10 kJ.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
15
The graph here represents a p-V diagram where the x axis is the volume in units of 10-3 m3 and the y axis is the pressure in units of 105 N/m2. The work done by the gas as it contracts from (p2, V2) to (p1, V1) along the path indicated is
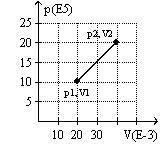
A) 30 kJ.
B) 10 kJ
C) -30 kJ.
D) 10 kJ.

A) 30 kJ.
B) 10 kJ
C) -30 kJ.
D) 10 kJ.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
16
The graph here represents a p-V diagram where the x axis is the volume in units of 10-3 m3 and the y axis is the pressure in units of 105 N/m2. The work done by the ideal gas as it follows the path sequence (p1,V1) to (p2,V2) to (p3,V3) to (p1,V1) along the lines indicated
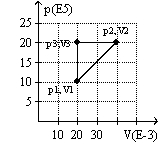
A) is 0 J.
B) is 10 kJ.
C) is -10 kJ.
D) cannot be determined from the given information.

A) is 0 J.
B) is 10 kJ.
C) is -10 kJ.
D) cannot be determined from the given information.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
17
The graph here represents a p-V diagram where the x axis is the volume in units of 10-3 m3 and the y axis is the pressure in units of 105 N/m2. The work done by the ideal gas as it follows the path sequence A to B to C to D back to A along the lines indicated is
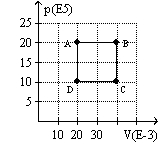
A) 80 kJ.
B) -80 kJ.
C) 20 kJ.
D) -20 kJ.

A) 80 kJ.
B) -80 kJ.
C) 20 kJ.
D) -20 kJ.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
18
Given a cyclic process on a p-V diagram, the area enclosed by the paths is
A) the change in the internal energy.
B) the work done by the system.
C) the net exchanged heat.
D) none of the above.
A) the change in the internal energy.
B) the work done by the system.
C) the net exchanged heat.
D) none of the above.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
19
In an adiabatic expansion of an ideal gas, 150 J of work is performed on the surroundings. The change in the internal energy of the gas is
A) 0 J.
B) 150 J.
C) -150 J.
D) unknown; not enough information is given to answer the question.
A) 0 J.
B) 150 J.
C) -150 J.
D) unknown; not enough information is given to answer the question.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
20
In an adiabatic expansion of an ideal gas, 150 J of work is performed on the surroundings. The heat added or removed from the gas is
A) 0 J.
B) 150 J.
C) -150 J.
D) unknown; not enough information is given to answer the question.
A) 0 J.
B) 150 J.
C) -150 J.
D) unknown; not enough information is given to answer the question.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
21
During an isothermal process involving an ideal gas, 150 J of heat is removed from the system. The change in the internal energy of the gas is
A) 0 J.
B) 150 J.
C) -150 J.
D) unknown; not enough information is given to answer the question.
A) 0 J.
B) 150 J.
C) -150 J.
D) unknown; not enough information is given to answer the question.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
22
During an isothermal process involving an ideal gas, 150 J of heat is removed from the system. The work done by the gas is
A) 0 J.
B) 150 J.
C) -150 J.
D) unknown; not enough information is given to answer the question.
A) 0 J.
B) 150 J.
C) -150 J.
D) unknown; not enough information is given to answer the question.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
23
A heat engine has an efficiency of 0.25 when it receives 250 J of heat. The amount of work done by the engine is
A) 0 J.
B) 63 J.
C) 190 J.
D) 250 J.
A) 0 J.
B) 63 J.
C) 190 J.
D) 250 J.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
24
A heat engine has an efficiency of 0.25 when it receives 250 J of heat. The amount of heat exhausted by the engine is
A) 0 J.
B) 63 J.
C) 190 J.
D) 250 J.
A) 0 J.
B) 63 J.
C) 190 J.
D) 250 J.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
25
For each 150 Btu a heat engine absorbs, the system exhausts 64 Btu. The efficiency of the engine is
A) 0.43.
B) 0.57.
C) 1.3.
D) 2.3.
A) 0.43.
B) 0.57.
C) 1.3.
D) 2.3.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
26
For each 150 Btu a heat engine absorbs, the system exhausts 64 Btu. The amount of work done by the engine for each 150 Btu absorbed is
A) 0 Btu.
B) 64 Btu.
C) 86 Btu.
D) 150 Btu.
A) 0 Btu.
B) 64 Btu.
C) 86 Btu.
D) 150 Btu.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
27
The Carnot efficiency of an engine that operates between the temperatures of 20°C and 50°C is
A) 0.093.
B) 0.90.
C) 0.40.
D) 0.60.
A) 0.093.
B) 0.90.
C) 0.40.
D) 0.60.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
28
The efficiency of a particular Carnot cycle is 100%. The difference in the operating temperatures (in Kelvin) of the reservoir
A) is zero.
B) is equal to the temperature of the hot reservoir.
C) is equal to the temperature of the cold reservoir.
D) cannot be determined from the information given.
A) is zero.
B) is equal to the temperature of the hot reservoir.
C) is equal to the temperature of the cold reservoir.
D) cannot be determined from the information given.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
29
The entropy of a closed system will
A) always increase.
B) always decrease.
C) increase or remain the same.
D) decrease or remain the same.
E) always remain the same.
A) always increase.
B) always decrease.
C) increase or remain the same.
D) decrease or remain the same.
E) always remain the same.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
30
When a vapor condenses, the entropy of the gas
A) increases.
B) decreases.
C) remains the same.
D) equals zero.
A) increases.
B) decreases.
C) remains the same.
D) equals zero.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
31
A heat engine works between two heat reservoirs with temperatures of 20°C and 100°C. In one cycle the engine produces 35 kJ of work while expelling 85 kJ of heat. The change in entropy is
A) 30 J/K.
B) 100 J/K.
C) 200 J/K.
D) 300 J/K.
A) 30 J/K.
B) 100 J/K.
C) 200 J/K.
D) 300 J/K.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
32
The change in entropy when 55 g of water (the latent heat of vaporization of water is 22.6 * 105 J/kg) at 100°C is turned into steam at 100°C is
A) 55 J/K.
B) 111 J/K.
C) 333 J/K.
D) 444 J/K.
A) 55 J/K.
B) 111 J/K.
C) 333 J/K.
D) 444 J/K.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
33
The change in entropy when 55 g of ice at 0°C melts (the heat of fusion is 3.34 * 105 J/kg) to water at 0°C is
A) 0 J/K.
B) 67 J/K.
C) -67 J/K.
D) infinite.
A) 0 J/K.
B) 67 J/K.
C) -67 J/K.
D) infinite.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
34
An ideal gas is compressed at a constant temperature of 27°C, during which 900 J of work is done on the gas. The change in entropy of the gas is
A) 33 J/K.
B) -33 J/K.
C) 3 J/K.
D) -3 J/K.
A) 33 J/K.
B) -33 J/K.
C) 3 J/K.
D) -3 J/K.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
35
1.0 mole of an ideal gas is in a container with a volume V1 at a pressure p1 and temperature T1. The gas is slowly compressed isothermally until the final volume is (1/4)V1. The change in entropy is
A) 0 J\K
B) -11 J\K
C) -47 J\K
D) 47 J\K
A) 0 J\K
B) -11 J\K
C) -47 J\K
D) 47 J\K

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
36
A container of 1.5 kg of water with a specific heat of 4186 J/(kg·°C) is heated from 25°C to 95°C. The change in the entropy of the water is
A) 1300 J/K.
B) 21 J/K.
C) 17 J/K.
D) 4.0 J/K.
A) 1300 J/K.
B) 21 J/K.
C) 17 J/K.
D) 4.0 J/K.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
37
200 J of work is expended in the adiabatic expansion of a gas. The change in the internal energy of the gas is
A) 0 J
B) 200 J.
C) -200 J
D) Hold on! Not enough information is given to solve the problem.
A) 0 J
B) 200 J.
C) -200 J
D) Hold on! Not enough information is given to solve the problem.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
38
200 J of work is expended in the adiabatic expansion of a gas. The amount of heat that enters the system is
A) 0 J
B) 200 J.
C) -200 J
D) Hold on! Not enough information is given to solve the problem.
A) 0 J
B) 200 J.
C) -200 J
D) Hold on! Not enough information is given to solve the problem.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
39
Two Carnot cycles have the same efficiency. The first operates between Th = 200°C and Tc = 100°C and the second operates with a hot reservoir of Th = 400°C. The lower reservoir's temperature is
A) 200°C.
B) 258°C.
C) 315°C.
D) 531°C.
A) 200°C.
B) 258°C.
C) 315°C.
D) 531°C.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
40
A Carnot air conditioner with CP = 8 uses 10 kW of power. The heat expelled to the outdoors is
A) 10 kW.
B) 30 kW.
C) 80 kW.
D) 90 kW.
A) 10 kW.
B) 30 kW.
C) 80 kW.
D) 90 kW.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
41
A heat pump with a coefficient of performance of 10 used 50 kW of power. The heat delivered to the house is
A) 5 kW.
B) 50 kW.
C) 60 kW.
D) 500 kW.
A) 5 kW.
B) 50 kW.
C) 60 kW.
D) 500 kW.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
42
The coefficient of performance, CP, for a heat pump is defined as
A) Q1/W.
B) W/Q1.
C) Q2/W.
D) W/Q2.
A) Q1/W.
B) W/Q1.
C) Q2/W.
D) W/Q2.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
43
The coefficient of performance, CP, for an air conditioner or refrigerator is defined as
A) Q1/W.
B) W/Q1.
C) Q2/W.
D) W/Q2.
A) Q1/W.
B) W/Q1.
C) Q2/W.
D) W/Q2.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
44
The temperature of 7.6 kg of water is raised from 35°C to 55°C. Given that the specific heat of water is 4186 J/(kg·°C), the change in the entropy is
A) 14,000 J/K.
B) -14,000 J/K.
C) 2000 J/K.
D) -2000 J/K.
A) 14,000 J/K.
B) -14,000 J/K.
C) 2000 J/K.
D) -2000 J/K.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
45
An ideal diatomic gas undergoes a two-step process. The process begins at a pressure pi and volume Vi. At the end of the two-step process pf = 1.5pi and Vf = 3.0Vi. The first step is an isothermal expansion to a volume V', followed by an adiabatic compression to the final volume. The intermediate volume V', in terms of the initial volume is
A) 1130Vi.
B) 13Vi.
C) 3.0Vi.
D) 130Vi.
A) 1130Vi.
B) 13Vi.
C) 3.0Vi.
D) 130Vi.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
46
The efficiency of a Carnot cycle is 0.20 when operating with a hot reservoir of 93°C. If the temperature of the cold reservoir is maintained, the temperature of the hot reservoir necessary to double the efficiency is
A) 47°C.
B) 186°C.
C) 215°C.
D) 488°C.
A) 47°C.
B) 186°C.
C) 215°C.
D) 488°C.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
47
An ideal gas is heated in a closed container. The net work done on the system
A) is zero.
B) is positive.
C) is negative.
D) depends on the gas in the container.
A) is zero.
B) is positive.
C) is negative.
D) depends on the gas in the container.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
48
During an isobaric (constant pressure) expansion, the volume of an ideal gas increases from 0.15 m3 to 0.35 m3. The pressure during the expansion is 150 kPa. The work done by the system is
A) -30 kJ.
B) 30 kJ.
C) 750 kJ.
D) -750 kJ.
A) -30 kJ.
B) 30 kJ.
C) 750 kJ.
D) -750 kJ.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
49
During an adiabatic expansion
A) Q > 0 and W = 0.
B) Q < 0 and W < 0.
C) Q = 0 and W > 0.
D) Q = 0 and W < 0.
A) Q > 0 and W = 0.
B) Q < 0 and W < 0.
C) Q = 0 and W > 0.
D) Q = 0 and W < 0.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
50
The metric unit associated with the efficiency of a heat engine is
A) joule.
B) kelvin.
C) joule/kelvin.
D) none of the above.
A) joule.
B) kelvin.
C) joule/kelvin.
D) none of the above.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck


