Exam 21: Thermodynamics
Exam 1: Space, Time, and Mass45 Questions
Exam 2: Motion Along a Straight Line51 Questions
Exam 3: Vectors50 Questions
Exam 4: Motion in Two and Three Dimensions50 Questions
Exam 5: Newtons Laws of Motion78 Questions
Exam 6: Further Applications of Newtons Laws50 Questions
Exam 7: Work and Energy51 Questions
Exam 8: Conservation of Energy50 Questions
Exam 9: Gravitation50 Questions
Exam 10: Systems of Particles46 Questions
Exam 11: Collisions50 Questions
Exam 12: Rotation of a Rigid Body50 Questions
Exam 13: Dynamics of a Rigid Body51 Questions
Exam 14: Statics and Elasticity50 Questions
Exam 15: Oscillations49 Questions
Exam 16: Waves51 Questions
Exam 17: Sound50 Questions
Exam 18: Fluid Mechanics50 Questions
Exam 19: The Ideal Gas50 Questions
Exam 20: Heat49 Questions
Exam 21: Thermodynamics50 Questions
Exam 22: Electric Force and the Electric Charge48 Questions
Exam 23: The Electric Field50 Questions
Exam 24: Gauss Law49 Questions
Exam 25: Electrostatic Potential and Energy52 Questions
Exam 26: Capacitors and Dielectrics40 Questions
Exam 27: Currents and Ohms Law50 Questions
Exam 28: Direct Current Circuits52 Questions
Exam 29: Magnetic Force and Field49 Questions
Exam 30: Charges and Currents in Magnetic Fields51 Questions
Exam 31: Electromagnetic Induction48 Questions
Exam 32: Alternating Current Circuits50 Questions
Exam 33: Electromagnetic Waves50 Questions
Exam 34: Reflection, Refraction, and Optics45 Questions
Exam 35: Interference and Diffraction50 Questions
Exam 36: The Theory of Special Relativity51 Questions
Exam 37: Quanta of Light49 Questions
Exam 38: Spectral Lines, Bohrs Theory, and Quantum Mechanics51 Questions
Exam 39: Quantum Structure of Atoms, Molecules, and Solids51 Questions
Exam 40: Nuclei46 Questions
Exam 41: Elementary Particles and Cosmology48 Questions
Select questions type
A heat engine produces a power output of 1.5 * 105 W when it receives 50.0 MJ of heat per minute. The efficiency of the engine is
Free
(Multiple Choice)
4.9/5  (39)
(39)
Correct Answer:
A
The change in entropy when 55 g of ice at 0°C melts (the heat of fusion is 3.34 * 105 J/kg) to water at 0°C is
Free
(Multiple Choice)
4.9/5  (40)
(40)
Correct Answer:
B
An ideal gas is heated in a closed container. The net work done on the system
Free
(Multiple Choice)
4.9/5  (36)
(36)
Correct Answer:
A
In an adiabatic expansion of an ideal gas, 150 J of work is performed on the surroundings. The heat added or removed from the gas is
(Multiple Choice)
4.9/5  (30)
(30)
A heat pump with a coefficient of performance of 10 used 50 kW of power. The heat delivered to the house is
(Multiple Choice)
4.9/5  (41)
(41)
In an adiabatic expansion of an ideal gas, 150 J of work is performed on the surroundings. The change in the internal energy of the gas is
(Multiple Choice)
4.8/5  (35)
(35)
The coefficient of performance, CP, for a heat pump is defined as
(Multiple Choice)
4.8/5  (35)
(35)
During an isobaric (constant pressure) expansion, the volume of an ideal gas increases from 0.15 m3 to 0.35 m3. The pressure during the expansion is 150 kPa. The work done by the system is
(Multiple Choice)
4.9/5  (32)
(32)
During an isothermal process involving an ideal gas, 150 J of heat is removed from the system. The work done by the gas is
(Multiple Choice)
4.9/5  (47)
(47)
For each 150 Btu a heat engine absorbs, the system exhausts 64 Btu. The efficiency of the engine is
(Multiple Choice)
4.9/5  (30)
(30)
The efficiency of a Carnot cycle is 0.20 when operating with a hot reservoir of 93°C. If the temperature of the cold reservoir is maintained, the temperature of the hot reservoir necessary to double the efficiency is
(Multiple Choice)
4.7/5  (35)
(35)
Two Carnot cycles have the same efficiency. The first operates between Th = 200°C and Tc = 100°C and the second operates with a hot reservoir of Th = 400°C. The lower reservoir's temperature is
(Multiple Choice)
4.9/5  (39)
(39)
The graph here represents a p-V diagram where the x axis is the volume in units of 10-3 m3 and the y axis is the pressure in units of 105 N/m2. The work done by the gas as it contracts from (p2, V2) to (p1, V1) along the path indicated is
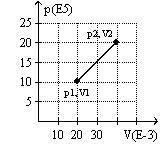
(Multiple Choice)
4.8/5  (31)
(31)
A container of 1.5 kg of water with a specific heat of 4186 J/(kg·°C) is heated from 25°C to 95°C. The change in the entropy of the water is
(Multiple Choice)
4.8/5  (36)
(36)
The graph here represents a p-V diagram where the x axis is the volume in units of 10-3 m3 and the y axis is the pressure in units of 105 N/m2. The work done by the gas as it expands from (p1, V1) to (p2, V2) along the path indicated is
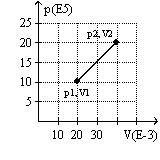
(Multiple Choice)
4.9/5  (31)
(31)
During an adiabatic process the change in the temperature of the system is
(Multiple Choice)
4.7/5  (44)
(44)
A Carnot air conditioner with CP = 8 uses 10 kW of power. The heat expelled to the outdoors is
(Multiple Choice)
5.0/5  (42)
(42)
Showing 1 - 20 of 50
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)